Article Text
Abstract
Objective There is a strong clinical association between IBD and primary sclerosing cholangitis (PSC), a chronic disease of the liver characterised by biliary inflammation that leads to strictures and fibrosis. Approximately 60%–80% of people with PSC will also develop IBD (PSC-IBD). One hypothesis explaining this association would be that PSC drives IBD. Therefore, our aim was to test this hypothesis and to decipher the underlying mechanism.
Design Colitis severity was analysed in experimental mouse models of colitis and sclerosing cholangitis, and people with IBD and PSC-IBD. Foxp3+ Treg-cell infiltration was assessed by qPCR and flow cytometry. Microbiota profiling was carried out from faecal samples of people with IBD, PSC-IBD and mouse models recapitulating these diseases. Faecal microbiota samples collected from people with IBD and PSC-IBD were transplanted into germ-free mice followed by colitis induction.
Results We show that sclerosing cholangitis attenuated IBD in mouse models. Mechanistically, sclerosing cholangitis causes an altered intestinal microbiota composition, which promotes Foxp3+ Treg-cell expansion, and thereby protects against IBD. Accordingly, sclerosing cholangitis promotes IBD in the absence of Foxp3+ Treg cells. Furthermore, people with PSC-IBD have an increased Foxp3+ expression in the colon and an overall milder IBD severity. Finally, by transplanting faecal microbiota into gnotobiotic mice, we showed that the intestinal microbiota of people with PSC protects against colitis.
Conclusion This study shows that PSC attenuates IBD and provides a comprehensive insight into the mechanisms involved in this effect.
- INFLAMMATORY BOWEL DISEASE
- Cholangitis
- PRIMARY SCLEROSING CHOLANGITIS
- COLONIC MICROFLORA
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is a strong clinical association between IBD and primary sclerosing cholangitis (PSC). However, currently it is unknown, if this association is due to common genetic polymorphisms or if PSC may drive IBD.
WHAT THIS STUDY ADDS
Unexpectedly, we found that PSC attenuates IBD. Mechanistically, PSC causes an altered intestinal microbiota composition, which promotes Foxp3+ Treg-cell expansion, and thereby protects against IBD.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We believe that our data build a basis for the development of new therapeutical strategies targeting the microbiota-Foxp3+ Treg-cell axis in IBD.
Introduction
IBD is characterised by chronic relapsing intestinal inflammation. The exact aetiology of IBD is not completely understood, but it is known that IBD is characterised by chronic inflammation, intestinal dysbiosis and mucosal barrier defects. Thus, one hypothesis is that IBD is a result of an aberrant immune response against intestinal bacteria in genetically susceptible individuals.1–3 There is a strong clinical association between IBD and primary sclerosing cholangitis (PSC), a chronic, cholestatic liver disease characterised by inflammation and fibrosis of the bile ducts inside and outside the liver. Approximately 60%–80% of people with PSC have concomitant IBD (from here on referred to as PSC-IBD).1 4 Conversely, only about 5% of people with IBD will develop PSC during their disease course.2 Notably, people suffering from PSC-IBD have a phenotype distinct from Crohn’s disease (CD) and Ulcerative colitis (UC), characterised by an overall milder IBD severity, a higher prevalence of right-sided predominant pancolitis, rectal sparing, backwash ileitis and an increased risk of developing colorectal neoplasia.3 5 Even for people with PSC without clinically manifested IBD, we have previously shown that a high proportion exhibits molecular signs of intestinal inflammation, characterised by immune cell infiltration and expression of proinflammatory cytokines in intestinal biopsies.6
The factors that contribute to the development of PSC-IBD are not yet understood. Previous studies suggest a critical role of CD4+ Foxp3+ regulatory T cells (Foxp3+ Treg) in IBD, as well as PSC.7–9 In line with these data, reduced Foxp3+ Treg-cell numbers and function were associated with single nucleotide polymorphisms in the IL2RA gene present in people with IBD and PSC.10–12 Interestingly, the microbiota plays a key role in the emergence of Foxp3+ Treg cells: microbiota-derived short-chain fatty acids (SCFAs) can facilitate the induction of Foxp3+ Treg cells both in in vitro and in animal models.13 14 Accordingly, aside from genetic predispositions in genes regulating Foxp3+ Treg-cell function, the intestinal microbiota has been suggested to be one of the contributing factors for the close association of IBD and PSC.15 Indeed, both IBD and PSC are characterised by intestinal dysbiosis. Moreover, direct comparisons revealed distinct microbiota compositions between these diseases.16–22 Thus, among others, the phylae Veillonella and Escherichia have been reported to be enriched in people with PSC-IBD compared with IBD alone, that are proposed to promote immune cell migration to the gut. In addition, bacteria of the Lachnospiraceae family which produce anti-inflammatory SCFAs were reported to be increased in people with PSC.23 However, it remains unclear, whether changes in the microbial composition caused by PSC lead to an altered Foxp3+ Treg-cell expansion and function that contributes to the phenotype of IBD in people with PSC.
Taken together, there is a clear connection between IBD and PSC. However, whether PSC increases the risk for IBD but attenuates its phenotype remains to be elucidated. In this study we combined cellular and microbial analyses from experimental mouse models of colitis and sclerosing cholangitis, biopsies and stool samples of people with PSC-IBD and IBD, and then performed human faecal microbiota transplantation (FMT) into gnotobiotic mice to decipher the impact of PSC on IBD.
Results
Experimental sclerosing cholangitis attenuates colitis severity and increases Foxp3+ Treg-cell frequency in mice
First, we aimed to test the connection between IBD and sclerosing cholangitis in experimental mouse models. To this end, Il10−/− mice, which develop spontaneous colitis24 were crossed to Mdr2−/− mice, a mouse model for experimental sclerosing cholangitis25 (figure 1A). As expected, Il10−/−Mdr2−/− mice, but not Il10−/− mice, developed sclerosing cholangitis based on increased transaminase AST and ALT levels, and fibrosis score (online supplemental figure S1A,B). Next, we assessed IBD severity. We found that Il10−/− and Il10−/−Mdr2−/− mice developed an overall mild colitis (figure 1B,C). Interestingly, Il10−/−Mdr2−/− mice with a concomitant experimental sclerosing cholangitis developed significantly reduced colitis compared with Il10−/− mice (figure 1C). However, there was little impact on weight, despite the differences observed in colitis severity using endoscopy. Of note, we aimed to induce a mild to moderate colitis severity in our experiments in order to limit the suffering of the animals. Thus, all mice showed a relatively mild weight loss, and we therefore may have not observed a difference. Moreover, while colonic CD4+ T-cell infiltration was comparable between the groups (figure 1D), the proportion of Foxp3+ Treg cells within the CD4+ T-cell population was significantly increased in the inflamed colon of Il10−/−Mdr2−/− compared with Il10−/− mice (figure 1D,E).
Supplemental material
Spontaneous colitis is reduced in mice with concomitant experimental primary sclerosing cholangitis in Il10−/−Mdr2−/− mice. (A) Graphical breeding scheme for generation of Il10−/− and Il10−/−Mdr2−/− littermates. Mice were bred under specific pathogen-free (SPF) conditions in the local mouse facility (MB1). After weening, litters were separated with respect to their genotype. At an age of 12 weeks, (B) body weight (n=22 Il10−/−, n=16 Il10−/−Mdr2−/−) and (C) colon inflammation was assessed by mouse colonoscopy (n=25 Il10−/−, n=13 Il10−/−Mdr2−/−), as described in material and methods. (D, E) Flow cytometry analysis of colon infiltrating CD4+ T-cell (n=17 Il10−/−, n=13 Il10−/−Mdr2−/−) and Foxp3+ Treg-cell frequencies of 12 weeks old mice (n=12 Il10−/−, n=10 Il10−/−Mdr2−/−). (F) Graphical breeding scheme for generation of Il10−/− and Il10−/−Mdr2−/− littermates bred in the presence of a colitogenic SPF microbiome (MB2) containing Helicobacter hepaticus, that was transferred to the founding animals. After weening, litters were separated with respect to their genotype. At the age of 12 weeks (G) body weight (n=8 Il10−/−, n=13 Il10−/−Mdr2−/−), (H) colonoscopy (n=8 Il10−/−, n=12 Il10−/−Mdr2−/−) and (I, J) frequencies of colon infiltrating CD4+ T cells and Foxp3+ Treg cells (n=6 Il10−/−, n=11 Il10−/−Mdr2−/−) were analysed. For statistical analysis, Mann-Whitney U test was performed.
The intestinal microbiota composition is known to impact colitis-susceptibility26. Therefore the colitis development we observed in Il10−/− mice under specific pathogen-free (SPF) conditions of the local mouse facility (referred to as MB1) was generally mild. To this end, we next aimed to determine spontaneous colitis development in Il10−/− mice bred in the presence of a colitogenic SPF microbiota, that showed a distinct beta diversity compared with MB1, including an enrichment of Helicobacter on genus level (referred to as MB2) (online supplemental figure S2A,B; figure 1F). As expected, the mice bred under MB2 conditions showed an overall increased susceptibility to developing colitis compared with mice with MB1 microbiota (figure 1C,H). Comparisons between Il10−/− and Il10−/−Mdr2−/− mice bred under MB2 conditions revealed no differences in body weight between the groups (figure 1G). However, colitis severity in Il10−/−Mdr2−/− mice with concomitant sclerosing cholangitis (online supplemental figure S1C,D) was significantly reduced compared with Il10−/− mice (figure 1H). Moreover, Il10−/−Mdr2−/− mice bred under MB2 condition showed reduced colonic CD4+ T-cell infiltration and increased Foxp3+ Treg-cell accumulation compared with Il10−/− mice (figure 1I,J).
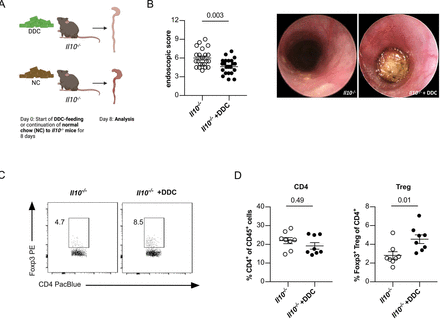
Next, we aimed to validate our observation in Il10−/−Mdr2−/− mice using a second model of experimental sclerosing cholangitis. To this end, we fed Il10−/− mice a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet.27 We used mice with the more colitogenic MB2 microbiota (figure 2A). DDC diet-induced sclerosing cholangitis in Il10−/− mice as determined by blood transaminase levels and fibrosis development (online supplemental figure S3A,B). In line with our results in Il10−/−Mdr2−/− mice, colitis severity and CD4+ T-cell infiltration in the inflamed colon of Il10−/− mice was attenuated under the DDC diet (figure 2B), and frequencies of colonic Foxp3+ Treg cells were increased compared with the regular chow diet (figure 2C,D).
Spontaneous colitis is reduced in Il10−/− mice with concomitant 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-mediated liver cholestasis. (A) Graphical scheme of the experimental setup. At an age of 6–8 weeks Il10−/− mice were gavaged with MB2. Four weeks after reconstitution, liver cholestasis was induced by 0.1% DDC feeding supplemented into the normal chow diet. After 8 days, (B) colonic inflammation was analysed by mouse colonoscopy (n=22 mice per group). (C, D) On day 9, mice were sacrificed and frequencies of colon infiltrating CD4+ T cells and Foxp3+ Treg cells were analysed using flow cytometry (8=mice per group). For statistical analysis Mann-Whitney U test was performed.
Thus, sclerosing cholangitis attenuates colitis severity in mouse models and is associated with an increased colonic Foxp3+ Treg-cell frequency.
Attenuated colitis severity in mice with sclerosing cholangitis is dependent on Foxp3+ Treg cells
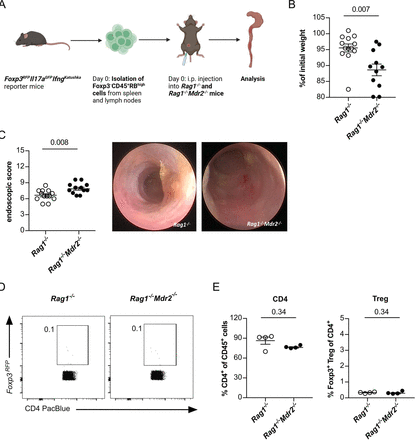
Since the reduced colitis severity was associated with a shift of CD4+ T-cell infiltration towards Foxp3+ Treg cells, we hypothesised that Foxp3+ Treg cells contribute to the limitation of colonic inflammation. Foxp3+ Treg cells are well known for their capacity to limit intestinal inflammation and restore immune homeostasis.28 Thus, to define the contribution of Foxp3+ Treg cells to the PSC-mediated attenuation of colitis, we used the T-cell transfer colitis model, in which Foxp3+ Treg cells are largely absent.29 To that end, we induced colitis in lymphopenic Rag1−/− and Rag1−/−Mdr2−/− mice, by transfer of naïve CD4+Foxp3−CD45RBhigh cells (figure 3A). As expected, Rag1−/−Mdr2−/− mice, but not Rag1−/− mice developed concomitant sclerosing cholangitis (online supplemental figure S4,B). Next, we assessed colitis severity and found it not to be attenuated in Rag1−/−Mdr2−/− mice, but in fact to be significantly increased compared with Rag1−/− based on weight loss and endoscopic score (figure 3B,C). As expected, no considerable Foxp3+ Treg-cell levels were detectable among CD4+ T-cell infiltrating cells (figure 3D,E).
Increased colitis manifestation in Rag1−/−Mdr2−/− mice after Foxp3−CD45RBhigh T-cell transfer. (A) Graphical scheme of the experimental setup. (B) At an age of 8–10 weeks Rag1−/− and Rag1−/−Mdr2−/− mice were gavaged with MB2. After 4 weeks of reconstitution, colitis was induced on transfer of flow cytometry sorted Foxp3−CD45RBhigh CD4+ T cells, isolated from Foxp3-RFP reporter mice. After 13 days of T-cell reconstitution, (B) weight loss and (C) colonic inflammation by colonoscopy were analysed (n=13 Rag1−/−, n=12 Rag1−/−Mdr2−/−). (D, E) At day 14, mice were sacrificed and frequencies of colon infiltrating CD4+ T cells and Foxp3+ Treg cells were analysed by flow cytometry in one of three experiments (n=4 Rag1−/− n=4 Rag1−/−Mdr2−/−). For statistical analysis Mann-Whitney U test was performed.
Taken together, the protective effect of sclerosing cholangitis on colitis appears to be dependent on the presence of Foxp3+ Treg cells.
FMT from Mdr2−/− mice into germ-free wild-type mice attenuates colitis severity
Alterations in the intestinal microbiota are a hallmark of IBD.30 Moreover, the intestinal microbiota is known to impact Foxp3+ Treg-cell differentiation and expansion.31 We therefore hypothesised that sclerosing cholangitis may alter the intestinal microbiota, and thus, reduce colitis severity. In order to test this hypothesis, we profiled the microbiota of stool samples collected from mice suffering from colitis alone (eg, Il10−/− mice and Rag1−/− mice on colitis induction via transfer of CD45Rbhigh cells) and with concomitant sclerosing cholangitis (eg, Il10−/−Mdr2−/− mice and Rag1−/−Mdr2−/− mice on colitis induction via transfer of CD45Rbhigh cells) (online supplemental figure S5). Comparison of beta diversities revealed clustering with some overlap of both experimental groups (online supplemental figure S5A,C), although a spread of samples between the groups was detected in both models. Of note, on the genus level, we found several taxa that significantly differed in abundance between the groups in the transfer colitis model (online supplemental figure S5D), but only one taxon in the Il10−/− model (online supplemental figure S5B). Most notably, an enrichment of genera of the Lachnospiraceae family was found in stool samples of mice suffering from colitis with concomitant liver inflammation in transfer colitis (online supplemental figure S5D).
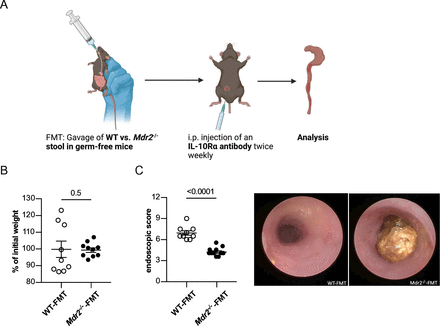
To decipher the functional relevance of the observed PSC-induced microbiota alterations on colitis severity, we next reconstituted germ-free wild-type mice with stool derived from mice with sclerosing cholangitis (Mdr2−/− mice) or without sclerosing cholangitis (wild-type mice), respectively, and induced colitis in these mice using a blocking anti-IL10Rα mAb32 (figure 4A). In accordance with our above-mentioned results (figure 1B,G), a mild weight loss was observed on colitis induction, that did not differ between the groups (figure 4B). However, endoscopic colitis severity was reduced in germ-free mice reconstituted with microbiota derived from Mdr2−/− mice compared with wild-type mice (figure 4C).
Reduced colitis severity in germ-free wild-type mice after transfer of Mdr2−/− microbiota. (A) Graphical scheme of the experimental procedure. In brief, faecal microbiota obtained from wild-type and Mdr2−/− mice, harbouring MB2 microbiome, was gavaged into germ-free wild-type mice. One day later, colitis was induced in these mice by intraperitoneal injection of 0.25 mg anti-IL10Rα antibody per mouse two times a week. After 13 days of colitis induction, (B) weight loss was determined and (C) colonic inflammation was analysed by colonoscopy (n=9 WT-FMT, n=10 Mdr2−/−-FMT). FMT, faecal microbiota transplantation; WT, wild-type.
Taken together, these results indicate that sclerosing cholangitis leads to alterations in the intestinal microbiota, in particular to an enrichment in genera of the Lachnospiraceae family. Furthermore, this altered intestinal microbiota of Mdr2−/− mice suffering from sclerosing cholangitis is protective against colitis, when compared with wild-type mice.
Colitis severity in germ-free mice is attenuated after FMT from people with PSC-IBD compared with IBD
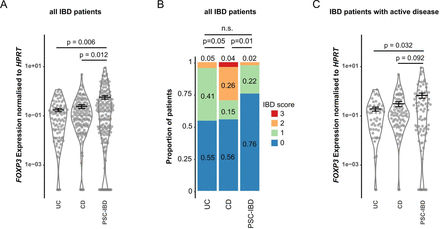
Based on the data obtained in the murine system, we next characterised FOXP3 mRNA expression levels in intestinal biopsies taken from a cohort of people with CD (n=29), UC (n=22) and PSC-IBD (n=41). We observed increased FOXP3 mRNA expression in the intestinal tissue of people with PSC-IBD compared with both, individuals with CD and UC (figure 5A). Within the cohort, we found milder IBD severity in people with concomitant PSC compared with people with CD and to a lesser extent to people with UC, as described previously (figure 5B).5 To account for this bias in disease severity, we next compared only those individuals with a clinically active disease as assessed by their physician. We again found an increased FOXP3 mRNA expression in the intestinal tissue of people with PSC-IBD compared with both individuals with CD and UC (figure 5C). Of note, the mean IBD score in all three groups was low and comparable (mean IBD-score for PSC-IBD: 0.48, CD: 0.52, UC: 0.78). To further test, if this decrease is biased by biopsies from a certain location, we plotted all biopsies from the same location for all patients. We found the same trend in all locations analysed: individuals with PSC-IBD having a higher FOXP3 mRNA expression compared with people with IBD without PSC (online supplemental figure S6A, online supplemental table S2). Next, we measured FOXP3 protein levels in tissue sections using immunohistochemistry. To this end we focused on biopsies from the terminal ileum and sigma/rectum. In line with the mRNA expression, we found an increased number of FOXP3+ cells in people with PSC-IBD compared with IBD without PSC (online supplemental figure S6B,C).
Supplemental material
FOXP3 mRNA expression and endoscopic IBD scoring reveal reduced clinical manifestation of IBD in people with primary sclerosing cholangitis (PSC-IBD). Description of a cohort including 29 people with Crohn’s disease (CD), 22 with Ulcerative colitis (UC) and 41 with PSC-IBD. (A) FOXP3 mRNA expression levels were analysed from intestinal biopsies taken from the terminal ileum, ascending and descending colon and sigma/rectum from every person. (B) IBD severity was determined based on CDAI (persons with CD) and Mayo score (all other persons). Both scores were merged into a unified IBD score (healthy/remission: 0, mild: 1, moderate: 2, severe: 3 points). (C) FOXP3 mRNA expression levels were analysed from intestinal biopsies taken from the terminal ileum, ascending and descending colon and sigma/rectum from every person with clinically active disease. To test for significance MLEM, post hoc Dunnett test was used for (A and C). Fisher’s exact test was used for (B).
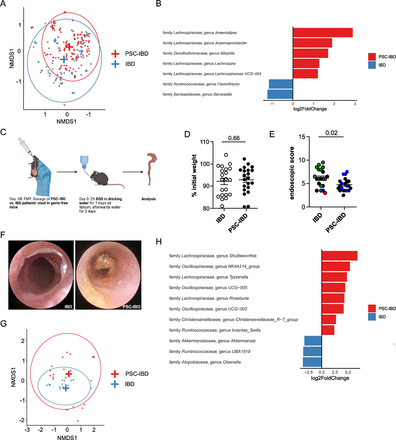
Next, we aimed to test whether the microbiota from people with PSC would protect against the development of concomitant IBD. Thus, we first performed microbiota profiling of mucosa-adherent bacteria isolated from intestinal biopsies derived from our IBD and PSC-IBD cohort, that has been partially published in Wittek et al, 2023. Sequencing of faecal microbiota revealed a large overlap, but also some differences in the microbiota composition of people with IBD and those with PSC-associated IBD. However, it is important to note that our study was not powered to decipher detailed microbiota differences between PSC-IBD and IBD as this point has been addressed by previous larger studies.23 33 Beta diversity comparison revealed a large overlap between people with IBD and PSC-IBD (figure 6A). In fact, on the genus level only a few taxa differed in abundance between both groups (figure 6B). Interestingly, genera of the Lachnospiraceae family were enriched in intestinal biopsies from people with PSC-IBD compared with IBD. To test the functional relevancy of this finding, we reconstituted germ-free wild-type mice with faecal microbiota samples derived from people with IBD or PSC-IBD and induced DSS colitis on reconstitution (figure 6C). Weight loss was comparable in both groups (figure 6D). However, the colitis severity as assessed by endoscopy was significantly reduced in mice reconstituted with faecal microbiota from people with PSC-IBD compared with IBD alone (figure 6E,F). To address whether ursodeoxycholic acid (UDCA) treatment mediates the observed effect, we performed a gnotobiotic mouse experiment. Specifically, a faecal microbiota transfer from healthy control (HC), without UDCA treatment, and primary biliary cholangitis (PBC) patients, with UDCA treatment, into germ-free mice was performed. People with PBC with mild cholestasis comparable to that of people with PSC-IBD were selected as cholestatic controls (online supplemental figure S7A,B). On engraftment, DSS-colitis was induced. A comparable colitis severity was observed between these groups, indicating that UDCA does not per se influence the colitis activity (online supplemental figure S7C,D). Next, we analysed whether the observed protection of these PSC-IBD-specific gnotobiotic mice is associated with an enrichment of genera of the Lachnospiraceae family on FMT. Microbiota profiling of donors (see online supplemental table S1 for the clinical information) and recipient mice was performed and can be found in online supplemental figure S8A. A Permanova analysis showed a significant contribution of disease, group (donor vs recipient) and donor on the variation observed in the data (online supplemental figure S8B). The abundance of bacteria in the different donors is comparable to a cross-sectional cohort (online supplemental figure S8C). When looking at the 10 highest abundant families, we observed that these were in most cases distributed similarly between recipients of the same donor, although at different proportions compared with the donor (online supplemental figure S8D). Beta diversities showed some overlapping of clusters representing each of the groups (figure 6G). Indeed, a strong enrichment of genera of the Lachnospiraceae family was detectable in faecal samples of mice that had been reconstituted with PSC-IBD stool compared with IBD stool (figure 6H).
Supplemental material
Colitis severity in germ-free mice is attenuated after FMT from people with primary sclerosing cholangitis and colitis (PSC-IBD), enriched for genera of the Lachnospiraceae family. Microbiota profiling was performed on mucosal tissue samples of our IBD and PSC-IBD cohort, as described in the material and methods. (A) PCoA of Bray-Curtis dissimilarities shows beta diversity across people with IBD and PSC-IBD. (B) Genera with significantly different abundance between people with IBD and PSC-IBD. (C) Graphical scheme of the protocol for faecal microbiota transplantation of stool derived from IBD or PSC-IBD patients into germ-free wild-type mice, and subsequent DSS colitis induction. After 9 days of colitis induction, (D) weight loss was determined and (E and F) colonic inflammation was analysed by colonoscopy (each dot represents one mouse). IBD activity of the donor is shown as: remission (black), mild (green), moderate (blue) and severe (red). (G and H) Microbiota profiling from stool samples collected from mice after reconstitution with stool samples from our IBD and PSC-IBD cohort. (D–H) n=21 mice transplanted with IBD stool; n=21 mice transplanted with PSC-IBD stool were used in four independent experiments.
In conclusion, these data indicate that PSC induces alterations of the intestinal microbiota, in particular an enrichment of genera of the Lachnospiraceae family, which in turn attenuate colitis susceptibility.
Discussion
In line with previous reports,3 5 we found that people with PSC-IBD present with milder colitis severity compared with people with IBD without PSC in our cohort. Likewise, we found a lower IBD susceptibility in a genetic (Mdr2−/−) and an induced (DDC-diet) mouse model of sclerosing cholangitis.
Alterations in the intestinal microbiota of people with PSC-IBD and IBD without PSC have been documented in various studies. These studies have yielded somewhat divergent findings,19 33 possibly due to variations in participant selection criteria, sampling locations and sample processing. Our study, along with several others, consistently identified an elevation in Lachnospiraceae among people with PSC-IBD compared with those with IBD without PSC.17 23 It could be possible that cholestasis, which is observed in people with PSC mediates the observed effects on the intestinal microbiota. In this case a similar effect should be observed in people and mouse models with cholestasis even in the absence of PSC. Further studies will be critical to address this point.
We observed that the protective effect of sclerosing cholangitis on colitis susceptibility was transferable on faecal microbiota transfer from Mdr2−/− mice and people with PSC-IBD into germ-free mice. Overall, we found genera of the Lachnospiraceae family to be abundant in the faecal samples of people with PSC used for the faecal microbiota transfer experiment. This finding is in line with a previous study by our group.6 Importantly, genera of the Lachnospiraceae family were over-presented in faecal samples after engraftment of the germ-free mice, supporting the notion that these could be involved in the protective effect. However, there is still the limitation that the number of donors may not fully capture the range of microbiota variability in people with PSC. In line with this finding, a previous publication has reported that Mdr2−/− mice treated with vancomycin, which reduced Lachnospiraceae and Clostridiaceae, had an increased liver pathology. Supplementation of these mice after antibiotic treatment with a 23 strain Lachnospiraceae consortium reduced histological liver inflammation and fibrosis.22 Conversely, in people with PSC, the Lachnospiraceae Blautia (genus), Lachnospiraceae bacterium 1_4_56FAA was negatively correlated with the Mayo risk score.22
Another important observation of this study is the association between increased Foxp3+ Treg-cell accumulation in the colon and over-representation of Lachnospiraceae in faecal samples. This has been observed in our mouse models of experimental sclerosing cholangitis with concomitant colitis, and in people with PSC-IBD compared with people suffering from IBD without PSC. Lachnospiraceae have indeed been associated with the production of SCFAs,34 35 which in turn have been linked to the induction of Foxp3+ Treg cells.35–38 Therefore, further assessment of SCFAs from faecal samples of our IBD and PSC-IBD cohort and mouse models of sclerosing cholangitis is required to test whether the enrichment in Lachnospiraceae is indeed associated with increased SCFA levels and subsequently increased Foxp3+ Treg-cell numbers.
One limitation of this study is that the role of Lachnospiraceae remains controversial. While some taxa produce butyrate, which can strengthen the intestinal barrier, others produce propionate, which can drive mucin degradation.34 More in-depth analysis of this family of bacteria in people with PSC-IBD, for example, through metagenomics, could help to identify which taxa are involved and how their metabolites could influence IBD development. Similarly, another publication39 showed an increase of Lachnospiraceae in faecal samples of Mdr2−/− mice. Transfer of the dysbiotic Mdr2−/− microbiota into healthy wild-type mice induced NLRP3 activation in the gut and the liver, which sustained liver injury and promoted disease progression. It would be important to further investigate the role of different taxa of Lachnospiraceae in the relationship of PSC and IBD.
Interestingly, a recent study identified Klebsiella pneumoniae in mesenteric lymph nodes of people with PSC, and also in faecal samples.40 Subsequent studies revealed that K. pneumoniae causes disruption in the epithelial barrier, resulting in the translocation of bacteria and subsequent inflammation in the liver. These discoveries emphasise how pathobionts contribute to dysfunction in the intestinal barrier and inflammation in the liver.40 Given the crucial role of the microbiome, it would be of interest to study whether the PSC microbiota can also modify complications of IBD in PSC, such as cancer risk.
The observation that people with PSC-IBD have a lower IBD activity on average, compared with people with IBD,6 is also reflected in our selected donors for the faecal microbiota experiments (online supplemental table S1). However, it appears that the observed protective effect was not linked to this difference. Of note, neither Mdr2-deficient mice nor control mice, which were used as donors for the FMT experiment, developed spontaneous colitis. This strengthens the observation that the IBD activity of the microbiota donor on the IBD susceptibility of the recipient does not play a key role for the observed protective effect.
Another interesting question that arose during this study is whether the protective effect observed is related to the UDCA treatment that people with PSC commonly receive. Thus, we compared colitis susceptibility of germ-free wild-type mice on transfer of faecal microbiota from PBC patients, who also commonly receive UDCA, to faecal microbiota from HCs. We could not find a difference between these two groups. In addition, we transferred faecal microbiota from Mdr2-deficient mice, which had not received UDCA, into germ-free mice. In this set of experiments, we also observed a protective effect of the microbiota from Mdr2-deficient mice compared with control mice on colitis susceptibility. Therefore, our data argue against a beneficial effect of the UDCA treatment on the IBD susceptibility after FMT of PSC-IBD microbiota. However, we were not able to compare cholestatic cohorts with or without UDCA treatment and therefore, we cannot exclude an additional effect of UDCA on colitis severity mediated by the microbiota composition as it has been shown recently by He et al.41
Interestingly, we found an increased FOXP3 mRNA and protein expression in the colon of people with active PSC-IBD, compared with active IBD without PSC. In addition, we identified an increased infiltration of Foxp3+ Treg cells in the inflamed colon of mice with concomitant sclerosing cholangitis in our mouse models. Interestingly, patients with genetic mutations42 in the FOXP3 gene, that have no or non-functional Treg cells, develop severe intestinal inflammation. Furthermore, adoptive transfer of autologous OVA-specific or polyclonal Treg cells has been shown to reduce CD and PSC-associated UC.43 Therefore, our data argue for the involvement of Foxp3+ Treg cells in the protective effect of PSC on IBD in humans and mice. This hypothesis is supported by our finding that the protective effect of liver cholestasis on colitis severity was not detectable in the CD4+CD45RBhigh T-cell transfer colitis model,44 45 in which Foxp3+ Treg cells are largely absent. One limitation of this experiment is that, although Foxp3+ Treg cells were depleted before the transfer into the recipient mice, there is the possibility of inducing peripheral Foxp3+ iTreg cells. However, colon infiltrating Foxp3+ Treg cells were hardly detectable in our study. Furthermore, the factors that control colonic Treg-cell accumulation during sclerosing cholangitis with concomitant colitis revealed that sclerosing cholangitis per se did not promote Treg-cell infiltration in the absence of intestinal inflammation. In fact, an increase in colonic Treg-cell accumulation was only observed in a colitogenic environment during sclerosing cholangitis. This finding is in line with a recent study by Shaw et al which showed that FOXP3+ Treg-cell frequencies gradually increase with colitis severity in intestinal biopsies of people with PSC-IBD.46 Nevertheless, potential differences in the suppressive capabilities of colonic FOXP3+ Treg cells from people with IBD and PSC-IBD have not been assessed in this study and by Shaw et al.46 Thus further studies will be essential to decipher the mechanism how PSC influences Foxp3+ Treg-cell expression and function in the setting of intestinal inflammation.
Overall, it remains to be elucidated what mechanism drives the increased accumulation of colonic Foxp3+ Treg cells during PSC-associated IBD. Beyond a participation of SCFAs in Foxp3+ Treg-cell differentiation in the colon, it is also tempting to speculate that the differentiation and expansion of Foxp3+ Treg cells already occurs in the cholestatic liver, and that consequently increased numbers of Foxp3+ Treg cells traffic from the liver to the colon. Indeed, increased Foxp3+ Treg-cell frequencies have been found in livers with different diseases like chronic viral hepatitis and hepatocellular carcinoma compared with healthy livers.47 Further studies will be essential to test these hypotheses.
Interestingly, it is well known that there are shared genetic risk loci between PSC and IBD, however it is also well established that the co-occurrence is far too extensive to be explained by genetics alone.48 Overall, our study provides novel insights into the relationship between PSC and IBD. We found that despite the common co-occurrence of both diseases, PSC can actually modify the severity of IBD to a better outcome. This effect is mediated by changes in the microbiota, which promotes the expansion of the Foxp3+ Treg-cell pool. A recently published report showed that IBD also ameliorates PSC.49 Therefore, our data suggest that disease in one organ, for example, the liver, may modify the disease in the other, for example, the intestine, in this case limiting the disease severity in both organs. Thus, we believe that our study might serve as a basis for further investigations on the molecular mechanisms underlying these processes, and could therefore lead to the discovery of novel therapeutic targets for PSC and IBD.
Supplemental material
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Ethical commission of the medical association Hamburg (PV4444, PV7106). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank Morsal Sabihi for carefully proof-reading the manuscript, and also Cathleen Haueis, Sandra Wende, Amanda Pidgornji and Saskia Domanig for technical support.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
TB, FS and MT are joint first authors.
PP and SH are joint senior authors.
Contributors TB, FS, MT and BS collaboratively conceived, designed and carried out most of the experiments, analysed the data, provided critical intellectual input and drafted the manuscript. RJ, SB, DR and AF performed experiments and analysed data. JK provided critical intellectual input and aided in drafting the manuscript. MB and SW performed histological analysis of colon and liver pathology. ML, TC and GS performed immunohistochemistry staining of FOXP3 in human. NG, SC and GT provided critical intellectual input. TB, PP and SH collaboratively conceived and designed most experiments, supervised the study, drafted the manuscript and provided critical intellectual input. SH is acting as guarantor.
Funding This work was supported by the Deutsche Forschungsgemeinschaft (CRU306 to SH and SC (290522633 and 426581255), SFB841 to SH). SH has an endowed Heisenberg-Professorship awarded by the Deutsche Forschungsgemeinschaft.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.