Article Text
Abstract
Objective Probiotic Lactococcus lactis is known to confer health benefits to humans. Here, we aimed to investigate the role of L. lactis in colorectal cancer (CRC).
Design L. lactis abundance was evaluated in patients with CRC (n=489) and healthy individuals (n=536). L. lactis was isolated from healthy human stools with verification by whole genome sequencing. The effect of L. lactis on CRC tumourigenesis was assessed in transgenic ApcMin/+ mice and carcinogen-induced CRC mice. Faecal microbiota was profiled by metagenomic sequencing. Candidate proteins were characterised by nano liquid chromatography-mass spectrometry. Biological function of L. lactis conditioned medium (HkyuLL 10-CM) and functional protein was studied in human CRC cells, patient-derived organoids and xenograft mice.
Results Faecal L. lactis was depleted in patients with CRC. A new L. lactis strain was isolated from human stools and nomenclated as HkyuLL 10. HkyuLL 10 supplementation suppressed CRC tumourigenesis in ApcMin/+ mice, and this tumour-suppressing effect was confirmed in mice with carcinogen-induced CRC. Microbiota profiling revealed probiotic enrichment including Lactobacillus johnsonii in HkyuLL 10-treated mice. HkyuLL 10-CM significantly abrogated the growth of human CRC cells and patient-derived organoids. Such protective effect was attributed to HkyuLL 10-secreted proteins, and we identified that α-mannosidase was the functional protein. The antitumourigenic effect of α-mannosidase was demonstrated in human CRC cells and organoids, and its supplementation significantly reduced tumour growth in xenograft mice.
Conclusion HkyuLL 10 suppresses CRC tumourigenesis in mice through restoring gut microbiota and secreting functional protein α-mannosidase. HkyuLL 10 administration may serve as a prophylactic measure against CRC.
- colorectal cancer
- colonic microflora
- probiotics
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Depleted beneficial microbes in the gut microbiota is associated with colorectal cancer (CRC) tumourigenesis.
Probiotic supplementation is known to confer health benefits to humans.
WHAT THIS STUDY ADDS
We identified that the abundance of a probiotic species Lactococcus lactis is reduced in patients with CRC from three independent cohorts.
A new L. lactis strain, HkyuLL 10, was successfully isolated from healthy human stools and verified by whole genome sequencing.
HkyuLL 10 supplementation suppressed tumourigenesis in two CRC mouse models, accompanied by alleviating gut microbial dysbiosis with enriched probiotic species.
We identified that α-mannosidase is the functional protein of HkyuLL 10 by nano liquid chromatography-mass spectrometry.
The protective function of α-mannosidase was demonstrated in human CRC cells and patient-derived organoids, and it also markedly reduced tumour growth in CRC xenograft mice.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
HkyuLL 10 exhibits protective function against CRC tumourigenesis in mice by restoring gut dysbiosis and secreting functional protein α-mannosidase.
Supplementation of HkyuLL 10 is a potential prophylactic measure against CRC in humans.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related motility and the third-most common cancer worldwide.1 To reduce CRC burden, it is important to identify prophylactic agents to prevent its development at early stage or reduce recurrence following disease remission. It is well-known that the gut microbiota plays crucial roles in colorectal tumourigenesis. A dysregulated microbiota could disrupt intestinal homeostasis and contribute to CRC development.2 Given its importance, modulating gut microbiota for the prevention and treatment of CRC is one of the major dogmas being investigated, and current methods of microbiota manipulation include dietary intervention, faecal microbiota transplantation and direct administration of probiotics.
Probiotics are beneficial bacteria exerting health benefits once consumed. Multiple probiotics have been reported to exhibit inhibitory effect against CRC through releasing specific proteins or metabolites. For instance, Streptococcus thermophilus secretes protein β-galactosidase to inactivate the oncogenic Hippo signalling pathway,3 whereas Lactobacillus gallinarum produces metabolite indole-3-lactic acid against CRC tumourigenesis in mice.4 For humans, studies showed that ingestion of prebiotic, probiotic and symbiotic supplements are associated with significant decrease in CRC occurrence.5 These findings therefore demonstrated the potential of probiotics as prophylactics against colorectal tumourigenesis.
Through multicohort metagenomic analysis, we previously identified the marked depletion of probiotics in patients with CRC, including Lactococcus lactis.6 L. lactis is a Gram-positive lactic acid-producing bacterium frequently used in dairy product fermentation, and has been considered safe for human consumption. Previous study has reported that two L. lactis strains could be exploited as a probiotic cocktail to promote colonisation of beneficial commensals and IgA-mediated immunomodulation in human intestines.7 Here, we investigated the role of L. lactis in the early prevention of CRC. We revealed the marked depletion of L. lactis in patients with CRC, and successfully isolated a new strain of L. lactis (nomenclated as ‘HkyuLL 10’) from healthy human stools. HkyuLL 10 exhibited robust antitumourigenic effect in vitro and in CRC mouse models. Through mass spectrometry characterisation, α-mannosidase (αMAN) was identified as the key protein responsible for the protection function of HkyuLL 10, while it could significantly inhibit tumour growth in CRC mouse xenografts.
Methods
Bacteria isolation and culture
HkyuLL 10, a new strain of L. lactis, was isolated from healthy human stools. Faecal samples were first homogenised in sterilised phosphate-buffered saline (PBS) and filtered through cell strainer with a pore size of 100 µm. For selection culture, the filtered faecal solution was spread on M17 agar plate with 1% lactose. Three other strains of L. lactis were purchased from DSMZ (#4366, #20175, #20250; Braunschweig, Germany). Escherichia coli strain MG1655 (ATCC 700926, Manassas, Virginia, USA), a non-pathogenic human commensal intestinal bacterium,8 was used as a bacterial control. Both HkyuLL 10 and E. coli were cultured in brain heart infusion (BHI) broth under aerobic conditions. Bacterial conditioned medium (CM) was collected by centrifugation at 5000 rpm for 30 min and purification by filters with a pore size of 0.22 µm.
CRC tumourigenesis mouse models
Male transgenic ApcMin/+ mice aged 6 weeks were randomly segregated to receive one of the three treatments: (1) BHI broth; (2) E. coli MG1655 or (3) HkyuLL 10. A carcinogen-induced CRC mouse model was also established. Male C57BL/6 conventional mice aged 5 weeks were intraperitoneally injected with a single dose of azoxymethane (AOM; 10 mg/kg) (Merck, Darmstadt, Germany), followed by 7-day supplementation of 1% dextran sodium sulfate (DSS; MP Biomedicals, Solon, Ohio, USA) in drinking water. Bacterial gavage was started 7 days after DSS administration. Mice were daily gavaged with 1×108 colony-forming unit of bacteria (E. coli or HkyuLL 10) resuspended in 100 µL of BHI broth. Mouse colonoscopy (Karl Storz Endoskope, Tuttlingen, Germany) was conducted to confirm tumour growth before sacrifice. ApcMin/+ mice and AOM/DSS-treated mice were respectively sacrificed 12 weeks and 17 weeks after treatment, and small intestine, colon and stools were examined.
CRC tumourigenesis germ-free mouse model
Male germ-free BALB/c mice aged 8 weeks were intraperitoneally injected with a single dose of AOM (10 mg/kg), followed by three cycles of 7-day supplementation of 1% DSS in drinking water. Each cycle was separated by 7 days of resting period without DSS administration. After DSS cycles were completed, mice were randomly segregated to receive bacterial gavage (1×107 colony-forming unit) once per week. Mouse colonoscopy was conducted to confirm tumour growth before sacrifice. Mice were sacrificed 7 weeks after treatment, and colon was examined.
CRC xenograft mouse model
Male nude mice aged 5 weeks were subcutaneously injected with human CRC cell line HCT116 or HT29 (4×106 cells in 100 µL of Matrigel; Corning, Corning, New York, USA) into both left and right lower back areas (two tumours per mouse). Mice were randomly separated to receive either PBS or αMAN (1 mg/mL in PBS; #M7257, Sigma-Aldrich, St. Louis, Missouri, USA) by subcutaneous and intratumoural injection (100 µL per tumour). Treatment was carried out every 3 days along with tumour size measurement. Mice were sacrificed 2–3 weeks after treatment.
Shotgun metagenomic sequencing and analysis
Our in-house metagenomes (patient with CRC=203, healthy individual=184)9 and two published metagenomic datasets (2019_ YachidaS cohort,10 patient with CRC=186, healthy individual=252; 2021_YangYZ cohort,11 patient with CRC=100, healthy individual=100) were retrieved to examine the abundance of L. lactis in human faecal samples. Genomic DNA of mouse stools (100 mg) was extracted by PowerSoil Pro Kit (Qiagen, Hilden, Germany), and subjected to shotgun metagenomic sequencing (Illumina HiSeq 2000 platform) performed by Novogene (Beijing, China). Raw reads were checked by KneadData (V.0.12.0) to ensure that data consisted of high-quality contamination-free microbial reads. Taxa were assigned to metagenomic clean reads using k-mer-based algorithms implemented in Kraken 2 (V.2.1.2) taxonomic annotation pipeline. After rarefying to the minimal library size (6 045 977 reads), alpha (Shannon and Chao1 index) and beta diversities (principal coordinate analysis (PCoA)) were analysed using R package phyloseq (V.3.16). Dissimilarity among microbial communities was calculated by permutational multivariate analyses of variance (ANOVA) with 1000 iterations using Bray-Curtis distance. Differential species were identified by multivariate model MaAsLin2, and only taxa with relative abundance >0.01 in at least one sample were retained. Microbes with p value <0.05 and adjusted p value <0.05 (false discovery rate corrected) were considered statistically significant.
Construction of αMAN-expressing E. coli mutant strain
The sequence of αMAN in HkyuLL 10 was introduced into E. coli MG1655 to construct a mutant strain with αMAN expression. In brief, αMAN gene from HkyuLL 10 was amplified by PCR using αMAN-attλ primers. The insert and attλ plasmid were then ligated and introduced to E. coli DH5α, followed by inoculation on Lysogeny brot (LB) agar with ampicillin at 37℃ overnight. After incubation, colonies were selected and the introduction of plasmid was confirmed by PCR using αMAN-cx primers. The selected plasmid was then transformed into E. coli MG1655 by electroporation. Successful insertion of αMAN sequence was validated by PCR using MG1655attλ primers. Primers used for bacterial engineering are listed in online supplemental table 1.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Statistical analyses
All results are shown as mean±SD. Mann-Whitney U test was used to compare the difference in numerical variables between two groups unless specified. One-way ANOVA was used to compare the difference in numerical variables among three groups. Repeated measures two-way ANOVA was used to compare different timepoints or stages between two groups. All statistical tests were performed using GraphPad Prism (V.9.0) or R language. Two-tailed p value <0.05 was considered statistically significant.
Additional methods are provided in online supplemental information.
Results
L. lactis is depleted in stools of patients with CRC
We first analysed the relative abundance of L. lactis in our in-house faecal metagenomic cohort.9 The abundance of L. lactis was significantly depleted in faecal samples of patients with CRC as compared with healthy individuals (p<0.0001; figure 1A). For validation, we retrieved two published faecal metagenomic datasets and confirmed the marked depletion of L. lactis in patients with CRC from 2019_ YachidaS cohort10 (p<0.01) and 2021_YangYZ cohort11 (p<0.0001), compared with healthy individuals.
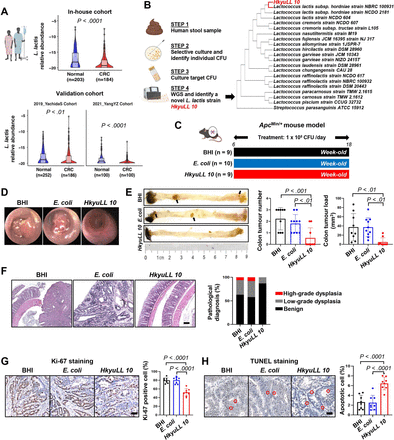
HkyuLL 10 protects against CRC tumourigenesis in ApcMin/+ mice. (A) Faecal abundance of Lactococcus lactis in patients with CRC and healthy individuals from an in-house cohort and two published metagenomic datasets. (B) Isolation of a novel L. lactis strain and phylogenetic tree of HkyuLL 10. (C) Experimental schematic of ApcMin/+ mice with daily supplementation of HkyuLL 10 or control (BHI broth or Escherichia coli). (D) Representative images of colonoscopy. (E) Colon morphology and tumour parameters, with black arrows indicating the visible tumours. (F) Representative colon H&E images and scoring of dysplasia. Scale bar=100 µm. (G, H) Representative images and scoring of Ki-67 staining (G) or TUNEL staining (H) in tumour tissues. Positively stained cells are circled in red. Scale bar=50 µm. BHI, brain heart infusion; CFU, colony-forming unit; CRC, colorectal cancer; WGS, whole genome sequencing.
A new L. lactis strain HkyuLL 10 protects against CRC tumourigenesis in ApcMin/+ mice
We successfully isolated L. lactis from faecal samples of healthy human individuals. Through whole genome sequencing, we identified that our isolated strain had a close phylogenetic relationship to L. lactis subspecies Hordniae strain NBRC 100931 but with different genomic sequence. The whole genome sequences of HkyuLL 10 and L. lactis Hordniae strain NBRC 100931 are provided in online supplemental data 1 and 2, respectively. Hence, we confirmed our discovery of a new L. lactis strain and nomenclated as ‘HkyuLL 10’ (figure 1B).
We evaluated the effect of HkyuLL 10 in ApcMin/+ mice, a transgenic mouse model of intestinal tumourigenesis, with daily gavage of BHI broth, E. coli or HkyuLL 10 (figure 1C). Visually smaller tumours were observed in HkyuLL 10-treated mice than control mice treated with BHI or E. coli under colonoscopy (figure 1D). After 12 weeks of treatment, mice were sacrificed and significant reduction in tumour number and tumour load was observed in the colon (figure 1E) and small intestine (online supplemental figure 3) of HkyuLL 10-treated mice, compared with control mice. Histological assessment showed that HkyuLL 10-treated mice had a lower percentage of low-grade dysplasia as compared with the two control groups (figure 1F). HkyuLL 10 supplementation also markedly reduced the amount of Ki-67 positive proliferating cells (figure 1G) and increased apoptotic cells (figure 1H) in tumour tissues of gavaged mice.
HkyuLL 10 protects conventional mice from carcinogen-induced CRC tumourigenesis
We then tested the tumour-suppressing effect of HkyuLL 10 in another CRC tumourigenesis mouse model chemically induced by AOM/DSS, with daily gavage of BHI, E. coli or HkyuLL 10 (figure 2A). Visually smaller tumours were observed in HkyuLL 10-treated mice than control mice treated with BHI or E. coli under colonoscopy (figure 2B). After 17 weeks of treatment, mice were sacrificed and we observed significant reduction in tumour number and tumour load in the colon of HkyuLL 10-treated mice (figure 2C). Histological assessment showed that HkyuLL 10-treated mice had a lower percentage of low-grade dysplasia as compared with the two control groups (figure 2D). HkyuLL 10 supplementation also significantly decreased Ki-67 positive proliferating cells (figure 2E) and increased apoptotic cells (figure 2F) in tumour tissues of gavaged mice. Together, the consistent findings between two mouse models indicated the protective role of HkyuLL 10 against CRC tumourigenesis in mice.
HkyuLL 10 protects conventional mice from carcinogen-induced CRC tumourigenesis. (A) Experimental schematic of AOM/DSS-treated conventional mice with daily supplementation of HkyuLL 10 or control (BHI broth or Escherichia coli). (B) Representative images of colonoscopy. (C) Colon morphology and tumour parameters, with black arrows indicating the visible tumours. (D) Representative colon H&E images and scoring of dysplasia. Scale bar=100 µm. (E, F) Representative images and scoring of Ki-67 staining (E) or TUNEL staining (F) in tumour tissues. Positively stained cells are circled in red. Scale bar=50 µm. AOM, azoxymethane; BHI, brain heart infusion; CFU, colony-forming unit; CRC, colorectal cancer; DSS, dextran sodium sulfate.
HkyuLL 10 protects germ-free mice from carcinogen-induced CRC tumourigenesis
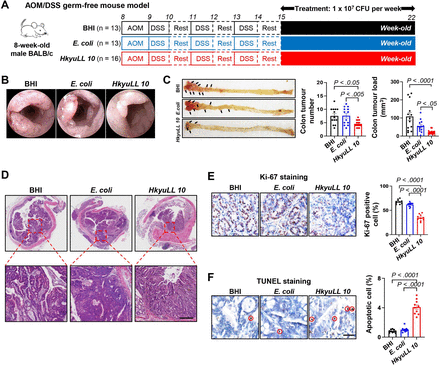
To evaluate the sole beneficial effect of HkyuLL 10, we supplemented BHI, E. coli or HkyuLL 10 to germ-free mice with AOM/DSS-induced CRC tumourigenesis (figure 3A). Visually smaller tumours were observed in HkyuLL 10-treated mice than control mice treated with BHI or E. coli under colonoscopy (figure 3B). After 7 weeks of treatment, mice were sacrificed and we found that HkyuLL 10 significantly decreased tumour number and tumour load in the colon of gavaged mice, compared with control (figure 3C). Histological assessment confirmed the occurrence of colon cancer in mice (figure 3D). Significantly decreased Ki-67 positive proliferating cells (figure 3E) and increased apoptotic cells (figure 3F) were observed in tumour tissues of HkyuLL 10-treated mice, compared with control. Hence, these findings demonstrated that HkyuLL 10 alone is adequate to suppress CRC tumourigenesis in mice with a depleted microbiota.
HkyuLL 10 protects germ-free mice from carcinogen-induced CRC tumourigenesis. (A) Experimental schematic of AOM/DSS-treated germ-free mice with weekly supplementation of HkyuLL 10 or control (BHI broth or Escherichia coli). (B) Representative image of colonoscopy. (C) Colon morphology and tumour parameters, with black arrows indicating the visible tumours. (D) Representative colon H&E images. Scale bar=100 µm. (E, F) Representative images and scoring of Ki-67 staining (E) or TUNEL staining (F) in tumour tissues. Positively stained cells are circled in red. Scale bar=50 µm. AOM, azoxymethane; BHI, brain heart infusion; CFU, colony-forming unit; CRC, colorectal cancer; DSS, dextran sodium sulfate.
HkyuLL 10 modulates gut microbiota with enriched probiotics in CRC mice
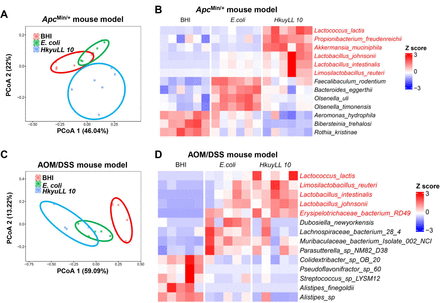
Since probiotics could confer benefits against colorectal tumourigenesis by modulating microbiota, we elucidated the impact of HkyuLL 10 on the gut microbiota by performing metagenomic sequencing on faecal samples of ApcMin/+ mice. β-Diversity analysis by PCoA showed that the microbial community in HkyuLL 10-treated mice was distinctively separated from the two control groups (figure 4A), implying that HkyuLL 10 could alter the composition of gut microbiota. A significant shift of microbial species was also observed in HkyuLL 10-treated mice, particularly including the enrichment of known probiotics such as Propionibacterium freudenreichii, Akkermansia muciniphila, L. johnsonii, Lactobacillus intestinalis and Limosilactobacillus reuteri (figure 4B).
HkyuLL 10 modulates gut microbiota with enriched probiotics in CRC mice. (A) Beta-diversity by PCoA based on Bray-Curtis dissimilarity matrix in faecal samples of ApcMin/+ mice. (B) Heatmap of differential bacterial species (p<0.05) in ApcMin/+ mice, compared with broth control and Escherichia coli bacterial control groups. (C) Beta-diversity by PCoA in faecal samples of AOM/DSS-treated mice. (D) Heatmap of differential bacterial species in AOM-DSS-treated mice. AOM, azoxymethane; BHI, brain heart infusion; CRC, colorectal cancer; DSS, dextran sodium sulfate; PCoA, principal coordinate analysis.
Metagenomic sequencing was also conducted on faecal samples of mice with AOM/DSS-induced CRC, and β-diversity analysis by PCoA showed that the microbial community in HkyuLL 10-treated mice was distinct from the two control groups (figure 4C). Similar to ApcMin/+ mice, HkyuLL 10 supplementation greatly altered the gut microbiota with enriched probiotics including L. reuteri, L. intestinalis and L. johnsonii in gavaged AOM/DSS-treated mice (figure 4D). These findings therefore implied that HkyuLL 10 could elevate the abundance of commensal probiotics against CRC tumourigenesis in mice. In addition, differential analysis identified the enrichment of HkyuLL 10 in both gavaged ApcMin/+ (figure 4B) and AOM/DSS-treated mice (figure 4D). For further verification, PCR detection and Sanger sequencing were performed using custom-designed primers for HkyuLL 10 and E. coli MG1655 (online supplemental table 2). The results confirmed the specific enrichment of HkyuLL 10 or E. coli in gavaged mice, respectively (online supplemental figure 2).
HkyuLL 10 inhibits growth of CRC cells and patient-derived organoids
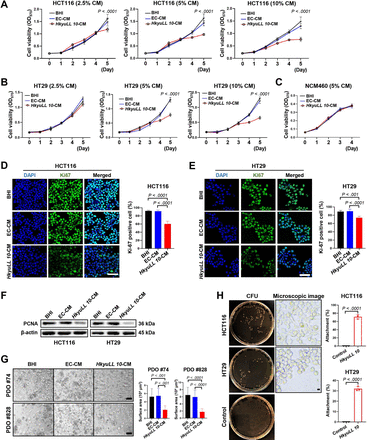
To understand the antitumourigenic mechanism of HkyuLL 10, we treated two human CRC cell lines (HCT116, HT29) with bacterial CM. The HkyuLL 10-CM at 2.5% concentration was sufficient to inhibit the viability of HCT116 cells, compared with broth control and the CM of E. coli (EC-CM) with the same concentration (p<0.0001; figure 5A). In comparison, HkyuLL 10-CM at 5% concentration was required to exhibit a suppressive effect on HT29 cells (p<0.0001; figure 5B). Meanwhile, 5% HkyuLL 10-CM posed no effect on the growth of human normal colon epithelial cell line NCM460 (figure 5C), suggesting that its inhibitory function was specific to CRC cells. The proportion of Ki-67-positive proliferating cells was also significantly reduced by 5% HkyuLL 10-CM in HCT116 (figure 5D) and HT29 (figure 5E) cells, compared with the two control groups. Consistently, the protein expression of proliferation marker proliferating cell nuclear antigen (PCNA) was downregulated in both CRC cell lines after HkyuLL 10-CM treatment (figure 5F). Moreover, we treated tumour organoids derived from two patients with CRC with 5% HkyuLL 10-CM, and observed a significant decrease in the growth of organoids as compared with broth control and EC-CM (figure 5G). Through bacteria-cell attachment assay, we further showed that HkyuLL 10 could attach to HCT116 and HT29 cells (both p<0.0001; figure 5H). Direct co-culture with HkyuLL 10 also suppressed the viability of HCT116 and HT29 cells, with no effect on normal NCM460 cells (online supplemental figure 3A,B). In addition, similar to HkyuLL 10-CM, the CM of three other L. lactis strains also significantly inhibited the viability of CRC cells, but not normal colon epithelial cells (online supplemental figure 3C,D), confirming the protective function of L. lactis in CRC.
HkyuLL 10 inhibits growth of CRC cells and patient-derived organoids. (A, B) Cell viability at OD570 of human CRC cell line HCT116 (A) or HT29 (B) under treatment of bacterial conditioned medium with different concentrations. (C) Cell viability at OD570 of human normal colon epithelial cell line NCM460 under treatment of 5% bacterial conditioned medium. (D, E) Representative images of Ki-67 immunofluorescent staining of HCT116 (D) or HT29 (E) cells. Scale bar=50 µm. (F) Protein expression of proliferation marker PCNA in CRC cells. (G) Representative images and size of tumour organoids derived from two patients with CRC under treatment of 5% bacterial conditioned medium. Scale bar=200 µm. (H) Macroscopic and microscopic images of HkyuLL 10 attachment to CRC cells. Scale bar=200 µm. BHI, brain heart infusion; CFU, colony-forming unit; CM, conditioned medium; CRC, colorectal cancer; DAPI, 4',6-diamidino-2-phenylindole; EC-CM, CM of Escherichia coli; OD, optical density; PDO, patient-derived organoid.
HkyuLL 10 produces antitumourigenic proteins with a molecular weight of >100 kDa
We next investigated molecules responsible for the antitumourigenic function of HkyuLL 10-CM. Bacteria can secrete various molecules including proteins and metabolites. Our results showed that the inhibitory effect of HkyuLL 10-CM on both HCT116 and HT29 CRC cell lines was completely abolished after heat inactivation (figure 6A). Heat-inactivated HkyuLL 10-CM also failed to restrain colony formation of CRC cells, as evidenced by the similar number of cell colonies observed among all groups (figure 6B). For validation, we treated HkyuLL 10-CM with proteinase K. Indeed, no inhibitory effect was demonstrated by proteinase K-treated HkyuLL 10-CM in HCT116 and HT29 cells, compared with proteinase K-treated broth control and EC-CM (figure 6C). Consistently, there was no difference in the number of cell colonies among all groups after proteinase K treatment (figure 6D). Hence, these results implied that the antitumourigenic molecules produced by HkyuLL 10 are proteins.
HkyuLL 10 produces antitumourigenic proteins with a molecular weight of >100 kDa. (A, B) Cell viability at OD570 (A) and colony formation (B) of CRC cells under treatment of heat-inactivated bacterial conditioned medium. (C, D) Cell viability at OD570 (C) and colony formation (D) of CRC cells under treatment of PK-treated bacterial conditioned medium. (E, F) Cell viability at OD570 of HCT116 (E) or HT29 (F) cells under treatment of bacterial conditioned medium with different molecular fractions. (G) Image of silver staining on bacterial conditioned medium, with arrows indicating five protein bands visible only in HkyuLL 10-CM >100 kDa. αMAN, α-mannosidase; BHI, brain heart infusion; CM, conditioned medium; CRC, colorectal cancer; EC-CM, CM of Escherichia coli; OD, optical density; PK, proteinase K.
To characterise the functional proteins, bacterial CM was separated into different fractions based on their molecular weight (30–50 kDa, 50–100 kDa, >100 kDa). We identified that only HkyuLL 10-CM with >100 kDa exhibited significant inhibitory effect on HCT116 cells (p<0.0001), while HkyuLL 10-CM with 30–50 kDa or 50–100 kDa had no impacts (figure 6E). Similar results were observed in HT29 cell line, where only HkyuLL 10-CM with >100 kDa could inhibit its viability (p<0.0001; figure 6F). Silver staining was therefore conducted on HkyuLL 10-CM with >100 kDa, and five distinct proteins bands with a molecular weight of >100 kDa were observed (figure 6G). Nano LC-MS analysis was then performed to characterise these five candidate proteins (online supplemental table 3), and αMAN was selected as the functional protein of HkyuLL 10. Through whole genome sequencing, the αMAN-encoding sequence in HkyuLL 10 genome was identified (online supplemental table 4).
α-Mannosidase inhibits growth of CRC cells and patient-derived organoids
The role of αMAN was tested in human CRC cells. We revealed that αMAN had similar activity in cell culture medium and bacteria culture broth (online supplemental figure 4A). αMAN significantly inhibited the viability of HCT116 (p<0.01) and HT29 (p<0.01) CRC cells in a concentration-dependent manner, whereas a much higher concentration of αMAN was required to reduce the viability of normal NCM460 cells (figure 7A and online supplemental figure 4B). αMAN treatment also markedly restrained the colony formation of CRC cells, compared with control (p<0.01; figure 7B). Moreover, a significant reduction in the size of CRC patient-derived organoids was observed after αMAN treatment (p<0.001; figure 7C). To confirm the functional role of αMAN, we introduced the sequence of αMAN into E. coli to construct a mutant strain with αMAN expression (online supplemental figure 4C). The CM of αMAN-expressing E. coli had similar inhibitory effect as HkyuLL 10-CM by suppressing the viability (figure 7D) and colony formation (online supplemental figure 4D) of HCT116 and HT29 cells. The growth of CRC patient-derived organoids was also significantly reduced by the CM of αMAN-expressing E. coli (figure 7E).
αMAN inhibits growth of CRC cells, patient-derived organoids and xenograft mice. (A) Cell viability at OD570 of human CRC cells (HCT116, HT29) and normal colon epithelial cells (NCM460) under treatment of αMAN (10 ng/mL). (B) Colony formation of CRC cells under treatment of αMAN. (C) Representative images and size of tumour organoids derived from two patients with CRC under treatment of αMAN. Scale bar=100 µm. (D) Cell viability at OD570 of CRC cells under treatment of 5% bacterial conditioned medium (>100 kDa). (E) Representative images and size of CRC patient-derived organoids under treatment of 5% bacterial conditioned medium (>100 kDa). (F) Tumour parameters of CRC xenograft mice bearing HCT116 tumours. (G, H) Representative images and scoring of Ki-67 staining (G) or TUNEL staining (H) in HCT116 tumours. Positively stained cells are circled in red. Scale bar=50 µm. (I) Tumour parameters of CRC xenograft mice bearing HT29 tumours. (J, K) Representative images and scoring of Ki-67 staining (J) or TUNEL staining (K) in HT29 tumours. αMAN, α-mannosidase; BHI, brain heart infusion; CRC, colorectal cancer; EC-αMAN-CM, αMAN-expressing Escherichia coli conditioned medium; OD, optical density; PBS, phosphate-buffered saline; PDO, patient-derived organoid.
α-Mannosidase suppresses tumour growth in CRC xenograft mice
We next validated the effect of αMAN in CRC xenograft mouse models bearing HCT116 tumours. Consistent with in vitro findings, αMAN exhibited robust antitumourigenic effect in HCT116 xenografts, as evidenced by the significant decrease in tumour growth compared with control mice receiving PBS (p<0.05; figure 7F). On sacrifice, macroscopic examination revealed the marked reduction in tumour volume (p<0.01) and tumour weight (p<0.001) in HCT116 xenografts treated with αMAN (figure 7F). Significantly decreased Ki-67-positive proliferating cells (p<0.001; figure 7G) and increased apoptotic cells (p<0.001; figure 7H) were also observed in HCT116 tumours of αMAN-treated xenografts, compared with control mice.
For validation, another CRC xenograft mouse model was established by injecting HT29 CRC cells. As expected, αMAN significantly suppressed tumour growth in these HT29 xenografts, compared with control mice receiving PBS (p<0.0001; figure 7I). On sacrifice, we observed the marked decrease in tumour volume and tumour weight in HT29 xenografts treated with αMAN (both p<0.001; figure 7I). Significantly decreased Ki-67-positive proliferating cells (p<0.001; figure 7J) and increased apoptotic cells (p<0.001; figure 7K) were also observed in HT29 tumours of αMAN-treated xenografts, compared with control mice. Together, our consistent in vitro and in vivo findings illustrated that HkyuLL 10 secretes protein αMAN to protect against CRC.
Discussion
In this study, we investigated the protective role of L. lactis against colorectal tumourigenesis. We first confirmed the faecal depletion of L. lactis in our in-house cohort and published metagenomic datasets of patients with CRC (figure 1). This result prompted us to hypothesise that L. lactis may play a pivotal role against CRC. Metagenomic analysis also suggested the presence of L. lactis in the gut microbiota of healthy individuals. Indeed, a strain of L. lactis was successfully isolated from healthy human stools. Through whole genome sequencing, we discovered that its genome is closely related but not completely identical to L. lactis subspecies Hordniae strain NBRC 100931, thus implying our identification of a new L. lactis strain (nomenclated as ‘HkyuLL 10’).
Here, we for the first time showed that L. lactis supplementation is sufficient to alleviate CRC tumourigenesis in mice. By supplementing HkyuLL 10 to mice with transgenic or carcinogen-induced CRC (figure 2), we observed that this novel L. lactis strain consistently abrogates CRC tumourigenesis in these mouse models. In general, probiotic supplementation is known to benefit human patients by alleviating and improving GI conditions such as diarrhoea and infection.12 Numerous preclinical studies also reported the marked inhibition of CRC by probiotics.3 4 13 For example, oral administration of Lactobacillus fermentum ZS40 was capable of mitigating intestinal inflammation and tumourigenesis in AOM/DSS-treated mice.14 Of note, HkyuLL 10 was able to suppress CRC tumourigenesis in germ-free mice (figure 3), indicating that HkyuLL 10 alone is sufficient to confer protective effect in microbiota-depleted mice. Collectively, these findings implied the promising prophylactic potential of L. lactis against CRC, as it could exhibit antitumourigenic function in a dysregulated microbiota, which frequently occurred in patients with CRC.15
Through faecal metagenomic sequencing, we identified that HkyuLL 10 could modulate the gut microbiota in CRC mice (figure 4). It is necessary for the gut microbiota to maintain a preferrable composition in the human body, whereas its alteration or dysregulation can affect various physiological functions including host immune response, digestion, metabolism and intestinal permeability.16 In particular, the balance of gut microbiota is substantially disrupted in colorectal tumourigenesis, resulting in the occurrence of microbial dysbiosis.15 Here, we found that HkyuLL 10 significantly increases the abundance of well-characterised commensal probiotics such as L. johnsonii and L. intestinalis in gavaged mice. Previous studies reported that the administration of these Lactobacilli could facilitate the reconstruction of a balanced microbiota with improved intestinal integrity in mice.17–19 Introducing a probiotic mixture containing six Bifidobacteria and Lactobacillus strains to postsurgery patients also lead to a significant reduction in pro-inflammatory cytokines and restoration in the intestinal microenvironment.20 Hence, these findings indicated that HkyuLL 10 suppresses CRC tumourigenesis, at least in part, through modulating and renovating the gut microbiota back to healthy and balanced conditions.
The results of in vitro biofunctional assays illustrated that the tumour-suppressing effect of HkyuLL 10 is attributed to its secreted proteins (figure 5). Subsequent characterisation by mass spectrometry identified that αMAN is the key functional component of HkyuLL 10 (figure 6). Probiotics produce different kinds of molecules including proteins and metabolites to confer health benefits,3 21 both of which could be used for tumour cell targeting.22 Here, we illustrated the antitumourigenic function of αMAN against the growth of CRC cells and patient-derived organoids, as well as subcutaneous tumour formation in xenograft mice (figure 7). αMAN is an enzyme expressed in the endoplasmic reticulum and Golgi apparatus of human cells for the processing and catabolism of N-linked glycans.23 Some gut commensal bacteria (eg, Bacteroides thetaiotaomicron, Enterococcus faecalis, Neobacillus novalis) also express αMAN to modulate or degrade yeast α-mannan and mannose-rich glycans.24–26 To ascertain the role of bacterial αMAN, we constructed a strain of E. coli that expressed the sequence of αMAN in HkyuLL 10. This mutant E. coli significantly inhibited the growth and proliferation of CRC cells and patient-derived organoids, thus confirming the tumour-suppressing function of HkyuLL 10-secreted αMAN. In general, N-glycans are closely associated with CRC as they can promote tumour growth, oncogenic signalling and metastasis.27 Several N-glycans were also identified as potential biomarkers of CRC.28 29 Given its ability to catabolise N-glycans, it is possible that αMAN degrades oncogenic N-glycans to protect against CRC or other cancers. For instance, αMAN could attenuate prostatic tumourigenesis by negatively regulating the oncogenic PTEN pathway.30 Notably, it is unlikely that αMAN alone is adequate to confer tumour-suppressing effect, as glycan degradation requires a variety of enzymes such as glucosidases, GlcNAc transferase and different members of mannosidases.31 Further investigation is suggested to decipher the role of other microbes-derived enzymes involved in glycan degradation in CRC in the future.
In conclusion, our research is a pioneering study which illustrates the complete adoption and effectiveness of the novel L. lactis strain HkyuLL 10 on CRC in multiple mouse models. HkyuLL 10 suppressed CRC tumourigenesis in mice by enriching gut commensal probiotics and secreting αMAN. The antitumourigenic function of αMAN was verified both in vitro and in vivo. Overall, our findings indicated that HkyuLL 10 administration is a potential prophylactic measure against colorectal tumourigenesis. Extensive research is imperative to ascertain the effect of HkyuLL 10 and evaluate whether its combination with other well-characterised probiotics could synergistically contribute to CRC prevention in humans.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
All animal studies were performed in accordance with guidelines approved by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong; and the Laboratory Animal Management and Use Committee of Shenzhen Jingtuo Biotechnology for germ-free mice.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
XD, HCHL and XK are joint first authors.
Contributors ACYS designed the study, performed the experiments and drafted the manuscript. XD, HCHL, XK and QL performed the experiments and revised the manuscript. XW, YLiu and LJ performed the experiments. YLu, WL and YD conducted bioinformatic analyses. AH-KC and KFT performed histological examination. JY designed and supervised the study and revised the manuscript. JY is the guarantor of this study.
Funding This project was supported by Research Talent Hub-Innovation and Technology Fund Hong Kong (ITS/177/21FP); RGC Research Impact Fund Hong Kong (R4032-21F); Shenzhen-Hong Kong-Macao Science and Technology Programme (Category C) Shenzhen (SGDX20210823103535016) and The Kingboard Precision Oncology Programme, CUHK.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.