Article Text
Abstract
Mounting evidence underscores the pivotal role of the intestinal barrier and its convoluted network with diet and intestinal microbiome in the pathogenesis of inflammatory bowel disease (IBD) and colitis-associated colorectal cancer (CRC). Moreover, the bidirectional association of the intestinal barrier with the liver and brain, known as the gut-brain axis, plays a crucial role in developing complications, including extraintestinal manifestations of IBD and CRC metastasis. Consequently, barrier healing represents a crucial therapeutic target in these inflammatory-dependent disorders, with barrier assessment predicting disease outcomes, response to therapy and extraintestinal manifestations.
New advanced technologies are revolutionising our understanding of the barrier paradigm, enabling the accurate assessment of the intestinal barrier and aiding in unravelling the complexity of the gut-brain axis. Cutting-edge endoscopic imaging techniques, such as ultra-high magnification endocytoscopy and probe-based confocal laser endomicroscopy, are new technologies allowing real-time exploration of the ‘cellular’ intestinal barrier. Additionally, novel advanced spatial imaging technology platforms, including multispectral imaging, upconversion nanoparticles, digital spatial profiling, optical spectroscopy and mass cytometry, enable a deep and comprehensive assessment of the ‘molecular’ and ‘ultrastructural’ barrier. In this promising landscape, artificial intelligence plays a pivotal role in standardising and integrating these novel tools, thereby contributing to barrier assessment and prediction of outcomes.
Looking ahead, this integrated and comprehensive approach holds the promise of uncovering new therapeutic targets, breaking the therapeutic ceiling in IBD. Novel molecules, dietary interventions and microbiome modulation strategies aim to restore, reinforce, or modulate the gut-brain axis. These advancements have the potential for transformative and personalised approaches to managing IBD.
- INTESTINAL BARRIER FUNCTION
- BRAIN/GUT INTERACTION
- INFLAMMATORY BOWEL DISEASE
- GASTROINTESTINAL CANCER
- ENDOSCOPY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- INTESTINAL BARRIER FUNCTION
- BRAIN/GUT INTERACTION
- INFLAMMATORY BOWEL DISEASE
- GASTROINTESTINAL CANCER
- ENDOSCOPY
WHAT IS ALREADY KNOWN ON THIS TOPIC
The intestinal barrier is pivotal in maintaining intestinal homeostasis and preventing harmful microbes and metabolites from entering the bloodstream.
Impairment of the intestinal barrier, often due to dietary factors and unfavourable microbiome composition, is closely linked to the development of inflammatory bowel disease (IBD) and colitis-associated cancer.
The intricate interplay of the gut-brain axis plays a role in the increased risk of extraintestinal manifestations in IBD and promotes metastasis in colorectal cancer (CRC).
WHAT THIS STUDY ADDS
Cutting-edge endoscopic techniques, like ultra-high magnification endocytoscopy and probe-based confocal laser endomicroscopy, enable real-time and in-depth assessment of the intestinal barrier down to the cellular level.
Innovative technology platforms hold the potential for spatial analysis of tissue ultrastructure, potentially advancing the ‘molecular’ barrier assessment.
Artificial intelligence enables advanced techniques to standardise and objectively assess barrier healing. Furthermore, it holds immense potential, although requiring further validation, in selecting therapeutic agents and predicting their success in clinical trials.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This new and precise assessment of the intestinal barrier can help connect the dots of the gut-brain axis, leading to a better understanding of IBD pathogenesis, preventing unfavourable outcomes and discovering new therapeutic targets.
Promising molecules targeting the gut-brain axis, including innovative oral nanomedicines, have been explored for personalised prevention and treatment of IBD and CRC.
Complementarily, dietary interventions offer potential adjunctive benefits in managing these disorders, with dietary sphingolipids showing promise in barrier modulation.
Introduction
Inflammatory bowel disease (IBD) represents multifaceted, chronic conditions primarily affecting the gastrointestinal tract with multi-systemic involvement.1 IBD significantly compromises patients’ quality of life, leading to adverse outcomes and high rates of complications, including the development of colitis-associated dysplasia and colorectal cancer (CRC),2 3 with subsequent mortality. Moreover, the burden on healthcare systems continues to escalate.4 Consequently, there is a pressing need to improve our understanding of IBD harnessing advanced technologies, fostering a more profound comprehension of its intricate pathogenic pathways, thereby paving the way for personalised medicine strategies.
Emerging research underscores the pivotal role of the intestinal barrier, composed of epithelial, immune and vascular components, in the development, progression and outcomes of IBD.5 The increased intestinal permeability, the first line of defence, has been increasingly associated with later development of IBD, contributing to its pathogenesis.6 The intestinal barrier integrity is dynamically influenced by the changing pattern of food, gut microbiota and microbiota-driven metabolites, as evidenced by the complementary ability of food and the rich community of intestinal microbes to modulate the barrier and the immune response.7 Moreover, the intestinal barrier exhibits a bidirectional and dynamic communication with the liver and the brain through the gut-brain axis, potentially elucidating and linking the distinct clinical presentations and complications of IBD.8 Deciphering this complex interconnection holds promise for enhancing disease management.
Recent findings have highlighted barrier healing as a promising therapeutic target in IBD, demonstrating its superiority compared with endoscopic and histological remission for predicting major adverse outcomes.9 Advances in real-time technologies can provide comprehensive, deep and integrated assessment of intestinal barrier healing. Advanced ultra-high magnification and resolution endoscopic techniques, such as probe-based confocal laser endomicroscopy (pCLE) and endocytoscopy, have demonstrated remarkable potential in assessing and quantifying real-time structural and functional barrier damage down to cellular components, with the ability to predict response to therapy and adverse clinical outcomes.10 Moreover, sophisticated spatial techniques, including multispectral imaging,11 nanoparticles (NPs)-based biophotonics,12 digital spatial profiling,13 optical spectroscopy14 and mass cytometry,15 hold the potential to offer detailed insights into the barrier’s heterogeneous molecular and ultrastructural aspects. In this advanced scenario, the application of artificial intelligence (AI) holds great promise for standardised, rapid and objective barrier assessment, enabling the seamless integration of in vivo and ex vivo ‘big data’, thereby facilitating improved assessment, stratification and outcome prediction.16 17
This narrative review comprehensively illustrates the latest cutting-edge evidence on the intestinal barrier and gut-brain axis in IBD, specifically providing the most recent and updated evidence on novel advanced technologies for cellular, molecular and ultrastructural barrier assessment. In contrast to traditional intestinal barrier assessment, mainly relying on challenging and laborious permeability tests, these newly available techniques, particularly when aided and integrated by AI, offer the potential for standardised assessment of barrier structure and function. This new translational, multimodal and personalised barrier assessment opens promising avenues for a better understanding of the gut-brain axis, aiming to identify effective therapeutic targets and agents. This paradigm represents the missing piece needed to realise precision medicine in IBD patients.
New mechanisms in IBD
Intestinal barrier
The outermost component of the intestinal barrier is the mucus layer.18 19 The mucus layer differs in composition according to the intestinal tract, reflecting the different exposures to microbes. It forms a single layer in the small intestine and a double-layered structure in the colon, consisting of a stirred mucus outer layer and a dense, non-stirred inner layer. The primary components are highly glycosylated gel-forming mucins intermingled with antimicrobial peptides and proteins.20 The mucus primarily acts as a physical barrier against microbes and harmful particles but exhibits tolerogenic activity by modulating immune cells in the lamina propria. Going deeper, we encounter the epithelial layer, composed of enterocytes and specialised cells, such as goblet cells, Paneth cells, enterochromaffin cells, tuft cells and stem cells. Notably, the enterocytes forming the intestinal monolayer are intricately interconnected and anchored to the basement membrane through protein complexes that ensure structural and functional integrity. These interconnections include tight junctions (TJs), adherens junctions (AJs) and desmosomes. The impairment of the mucus and epithelial layers has been linked to increased intestinal permeability, which can activate inflammation and carcinogenesis.21 Notably, proteins associated with the intestinal epithelial barrier, such as TJs and intestinal fatty acid binding protein, have shown potential as biomarkers for colitis-associated dysplasia and CRC detection.2 3
Microbes and molecules crossing mucus and epithelium face a third physical barrier within the lamina propria: the gut vascular barrier. This recently discovered barrier consists of fenestrated blood vessels that, under physiological conditions, prevent bacterial dissemination and passage of microbial-derived large molecules and dietary compounds into the portal and systemic circulations while allowing the passage of small molecules (up to 4 kDa).22 Advanced imaging techniques have recently established that the permeability of this barrier can be modulated by TJ and AJ proteins, such as claudin-1 and 5, zonula occludens-1 and junctional adhesion molecule-A, which strictly control paracellular transport from the gut lumen to the bloodstream.23 Additionally, the permeability of the gut vascular barrier can also be regulated by plasmalemma vesicle-associated 1 (also referred to as PV-1), an important protein involved in vascular fenestration. Interestingly, increased endothelial PV-1 detection has been observed in patients with ulcerative colitis (UC), suggesting a remodelling of transcellular permeability of the gut vascular barrier. Due to its direct connection with the bloodstream, vascular leakage can have knock-on effects on remote organs such as the liver and the brain.8 Thus, gut vascular barrier function emerges as a potential candidate for predicting colonic disorders’ outcomes and comorbidities, including pathogenesis.
The gut barrier is also characterised by an immune component, serving as a physiological barrier to control the passage of external microbes into the mucosa. This immune barrier comprises diverse immune and non-professional immune cells in the three main layers of the intestinal barrier (epithelium, lamina propria, endothelium) and efficiently guards the body against pathogens.24 Pattern-recognition receptors (PRRs), such as toll-like receptors, are notable elements of innate immunity. These are activated on recognising pathogen-associated molecular patterns (PAMPs) and mediate inflammatory pathways in the gut. A defective intestinal barrier compromising the first line of defence may result in an overdrive in adaptive immunity, resulting in inflammation.25
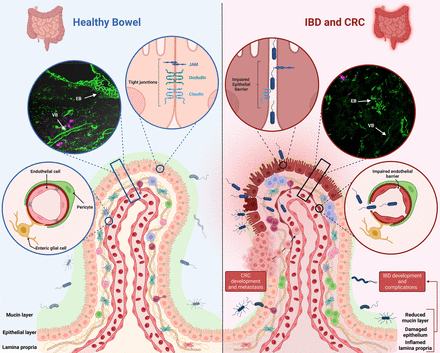
The main components of the intestinal barrier and their IBD-related impairment are schematically represented in figure 1.
Intestinal barrier components in health and inflammation. This figure schematically represents the intestinal barrier, depicting a healthy barrier on the left and an impaired barrier in inflammatory bowel disease (IBD) on the right. In healthy conditions, the mucus layer and the epithelial barrier, fortified by tight junctions (represented in the upper left circle), prevent microbial translocation. Other components of the intestinal barrier include immune cells in the lamina propria and the vascular barrier (represented in the lower left circle). An immunofluorescence image provided on the left shows staining for the tight junction ZO-1, demonstrating an intact epithelial barrier (EB) and vascular barrier (VB). In IBD, a compromised intestinal barrier, with a reduced mucin layer and disrupted tight junctions, permits harmful microbes to enter the lamina propria (as represented in the upper right circle). Microbes trigger inflammation and can translocate across the impaired vascular barrier into the bloodstream. Barrier impairment is associated with IBD and colorectal cancer (CRC) development and complications. The immunofluorescence image on the right, stained for ZO-1, illustrates a disrupted EB and VB in IBD. Created with ‘Biorender.com’.
Intestinal barrier-diet-gut microbiota: a new triangle
The structural and functional integrity of the intestinal barrier relies on its complex and still incompletely understood interplay with diet and gut microbiota with intestinal immune/stromal cells.
Dietary intake profoundly affects the gut barrier. Specific dietary components, including refined sugars, saturated fats and additives, compromise epithelial TJs, increase intestinal permeability and trigger inflammation.26 This diet-dependent immune modulation milieu involves another significant player, the gut microbiome, which completes the paradigm of the intestinal barrier-diet-microbiome triangle.
The gut microbiome, comprising an intricate consortia of bacteria, viruses and fungi resident in the bowel, critically mediates the effects of dietary substrates on the intestinal barrier. Westernised diets, characterised by high-fat and low-fibre contents, induce a dysbiosis shift in the microbiome, reducing bacterial diversity and enriching pro-inflammatory taxa like Proteobacteria and Actinobacteria.27 This microbial imbalance and aberrant glycan profiles disrupt TJs, increasing paracellular permeability and immune activation. The microbial genera Adlercreutzia, Clostridia UCG 014, Clostridium sensu stricto 1 and Colidextribacter, along with their associated pathways involved in the biosynthesis of glutamate, tryptophan and threonine, have been demonstrated to modulate gut barrier function.28 Furthermore, microbes regulate the immune response via the PRRs-PAMPs pathway, initiating a dialogue with macrophages and neutrophils, key cellular players in inflammation and subsequent organ damage, ultimately contributing to IBD pathogenesis.7 29 Similarly, the microbiome can play a significant role in CRC carcinogenesis, displaying both pro-carcinogenic and pro-metastatic features.30 A microbial signature, in which many bacterial species are usually colonisers of the oral cavity, has been described in CRC.31 Among these, Fusobacterium nucleatum, a pro-inflammatory gram-negative bacterium, can promote tumorigenesis by activating cancerous Wnt/β-catenin signalling in host cells and evading tumours from the immune system.32 More recently, the antigen-driven colonic inflammation, characterised by a pathogenic interleukin 17 (IL-17) signature, has shown to be a driver of the emergence of dysplasia in IBD.33
The crucial relationship between epithelial barrier and microbe-related modulation of the immune system in inflammatory immune-mediated disorders has led the European Academy of Allergy and Clinical Immunology to recognise two new mechanisms underlying allergic and autoimmune conditions, namely epithelial barrier defects and metabolic-induced immune dysregulation (type V and type VI hypersensitivity, respectively).34
Nonetheless, the interaction among the intestinal barrier, diet and the microbiome is also crucial for maintaining intestinal homeostasis. The Mediterranean diet produces beneficial microbial metabolites (postbiotics), which exhibit anti-inflammatory and antineoplastic properties, thereby exerting protective effects on the intestinal barrier and immune response.35 Short-chain fatty acids (SCFAs), including butyrate, propionate and acetate, are key modulators of inflammation, promoting immune cell function and enhancing barrier integrity. Additionally, metabolites like branched-chain amino acids (tryptophan, arginine, polyamines and taurine), indole compounds and omega-fatty acids (eicosapentaenoic acid, docosahexaenoic acid, α-linolenic acid) also possess anti-inflammatory and protective properties.27
The intricate interplay between the intestinal barrier, diet and microbiome underscores the crucial role of dietary intervention and microbiome modulation in preserving intestinal integrity and preventing gastrointestinal disorders. Therefore, targeting these components may offer a promising approach to personalised therapy in IBD aimed at restoring intestinal barrier integrity and immune homeostasis, as discussed in the New perspectives for IBD and CRC management section.
Gut-brain axis and liver-intestine axis
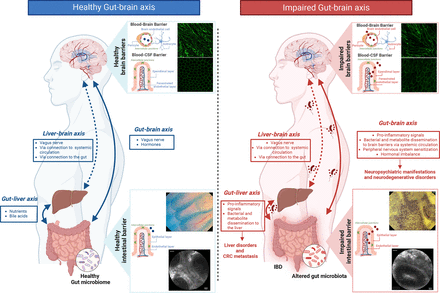
The dysfunction of the intestinal barrier-diet-microbiome network extends beyond local disease implications, significantly impacting the entire body. Communication channels within the body intricately connect the gastrointestinal tract and gut microbiota with the liver and brain, forming the so-called gut-brain axis (figure 2).18 This axis encompasses different routes, including inflammatory, hormonal, neural (via ascending and descending autonomic pathways) and microbial (via microbial translocations and secretion of microbial metabolites).
The gut-brain axis. This figure illustrates the complex interplay of the gut-brain axis in healthy conditions (left) and in inflammatory bowel disease (IBD) (right). In healthy conditions, the gut and microbiome interact bidirectionally with the brain through the vagus nerve and hormones and with the liver via nutrients and bile acids. A possible bilateral connection between liver and brain has also been hypothesised. Preserved intestinal barrier, as shown by endocytoscopy and confocal laser endomicroscopy images, maintains systemic homeostasis by preventing microbe dissemination. Simultaneously, intact blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB), as shown in immunofluorescence slides, hinder microbe and metabolite entry. In IBD, compromised intestinal barrier (epithelial damage at endocytoscope and fluorescein leakage at confocal laser endomicroscopy are shown) allows microbes and metabolite translocation into the bloodstream. Pro-inflammatory signals, microbes, metabolites and hormonal imbalance across the gut-brain and gut-liver axes led to neurological and liver disorders, and colorectal cancer metastasis. Correspondingly, impaired BBB and BCSFB (as evidenced by immunofluorescence images) exacerbate complications. Created with ‘Biorender.com’. CRC, colorectal cancer; CSF, cerebrospinal fluid.
Under physiological conditions, these communication channels ensure proper bidirectional communication between different body compartments and maintain global homeostasis. However, in pathological conditions such as IBD, these communication channels are altered, contributing over time to the worsening of disease severity, progression and the development of extraintestinal manifestations and complications.
A hallmark of IBD is intestinal disruption and an associated increase in intestinal permeability, commonly called ‘leaky gut’. This allows microbes to pass into systemic circulation, drive inflammation and impacting on various physiological and pathological processes. Growing evidence suggests a connection between ‘leaky gut’ and disorders in other organs, including those affecting the brain and liver, as well as the risk of metastasis in CRC.18
For instance, the gut microbiota and microbiota-derived metabolites reaching the brain due to intestinal barrier impairment can modulate the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB), which control the periphery and central nervous system exchanges and ensure proper brain homeostasis. Recent studies have shown that germ-free or antibiotic-treated mice display alterations in the integrity of the BBB and BCSFB.36 In addition, in colitis-induced mouse models, intestinal inflammation is associated with increased intestinal permeability, translocation of microbial components and structural and functional alterations of the BCSFB, which becomes more restrictive to external cues to protect the brain from inflammation.23 This impaired network may explain the heightened risk of developing neuropsychiatric manifestations in IBD, including fatigue, anxiety and depression, as well as neurodegenerative diseases like multiple sclerosis, Alzheimer’s and Parkinson’s disease.37 Recently, a correlation between IBD and pathological α-synuclein aggregation in the brain has been described, reinforcing the significance of gut-brain axis in initiating neurodegeneration.38 Additionally, patients with IBD are also known to display alterations in gut microbiota, which are also observed in patients with major depressive disorders and multiple sclerosis.39 Barrier impairment and altered gut microbiota can promote molecular imbalances and pro-inflammatory processes, increasing the prevalence of neurological comorbidities. The gut microbiota is known to modulate tryptophan metabolism, a molecular pathway related directly or indirectly to serotonin and melatonin synthesis.40 In this context, intestinal inflammation and microbial alteration in IBD have been associated with a neurotoxic shift leading to increased production of kynurenine, a metabolite with immunosuppressive properties and other tryptophan-derived neurotoxic compounds. These compounds, which have been previously associated with the pathogenesis of both major depressive disorder and multiple sclerosis, can circulate in the systemic circulation, modulate brain barriers, and reach the brain.41 The gut-brain connection has predominantly been explored through animal models, with only recent preliminary evidence using MRI in humans to assess differences in brain morphology and connectivity between IBD patients and controls. Significant differences in functional MRI observations of the left superior frontal gyrus, left cingulum and left supplementary motor area have been unveiled, confirming the neurological impairment in IBD.42 Similarly, changes in MRI-assessed choroid plexus volume and permeability have been associated with inflammatory activity in IBD, supporting the role of inflammation in modulating the BCSFB in humans.43
The microbiota-gut-liver axis has also recently emerged as a potent regulator of colitis-associated CRC progression. Due to its close connection with the gastrointestinal tract through the portal venous circulation and its involvement in nutritional processes, the liver represents CRC’s most common metastatic niche. Disruptions of the gut vascular barrier following CRC development led to the abnormal dissemination of microbes and microbial-derived metabolites into the liver. This creates a pre-metastatic niche, which attracts circulating tumour cells and promotes a pro-inflammatory environment.44 Furthermore, the high-fat dietary regimen-induced dysbiosis, leading to intestinal barrier disruption and ‘leaky gut’, is considered a pre-requisite for the development of liver disorders associated with IBD through the gut-liver axis. Those include non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and primary sclerosing cholangitis, as well as cirrhosis and hepatocellular carcinoma.8
Understanding the complex barrier-diet-microbiome interplay within the gut-brain axis is essential for elucidating systemic implications of IBD and developing targeted therapeutic interventions to treat and prevent complications.
Tools for assessing the intestinal barrier in IBD
The increasingly recognised significance of the intestinal barrier in IBD pathogenesis underscores the urgent need for its more comprehensive assessment, enabling a deeper understanding of the intricate interplay between the ‘leaky gut’ and the gut-brain axis. Furthermore, barrier healing represents a promising therapeutic target in IBD, and developing novel tools that can assess it in real-time, rapidly and objectively is imperative.
Traditionally, the assessment of barrier structure and function relied on indirect methods to evaluate its permeability, measuring the passage of solutes across the epithelium or evaluating transepithelial resistance (TER).5 The altered epithelial passage of solutes was assessed by measuring the urinary concentration of lactulose-mannitol and sucralose for the small intestine and the entire gastrointestinal tract, respectively. Other probes, such as sucrose and PEG-400 have also been used. Additionally, a decrease in TER and the compensatory increase in intestinal permeability indirectly suggest barrier impairment. However, these methods could provide only indirect information on the functional integrity of the barrier and could not reveal the morphological changes responsible for altered permeability. Therefore, there has been a focus on researching novel tools for a direct, comprehensive, cellular and molecular intestinal barrier assessment, aiming to predict outcomes and response to therapy, guiding a personalised approach in IBD.
Advanced endoscopic imaging techniques
The introduction of advanced ultra-high magnification endoscopic imaging techniques, notably pCLE and endocytoscopy, has revolutionised direct and real-time intestinal barrier assessment. These techniques offer histological-like examination, aiding in inflammation assessment, dysplasia characterisation and detection of clinically relevant barrier alterations, as demonstrated mainly in IBD.10 45
pCLE, combined with intravenous fluorescent agents, provides up to 1000-fold mucosal magnification and allows an in vivo dynamic structural and functional assessment of barrier integrity by identifying crypt morphology, vessel tortuosity and fluorescein leakage.46 A specific scoring system, called the Watson score, has been developed for the in vivo evaluation of small bowel barrier dysfunction in IBD using pCLE.47 This score, evaluates cell shedding and fluorescein luminal leakage and consist of three grades: I—normal; II—functional defect, with cell shedding confined to single cells per shedding site and visible fluorescein leakage in the intestinal lumen; III—structural defect, when the fluorescein leakage is associated with microerosions in any field. The Watson score demonstrated a good correlation with histology and offers innovative capabilities for assessing structural and functional barriers and predicting relapse in IBD.47 Recently, the intestinal barrier healing assessed through pCLE in Crohn’s disease (CD) and UC has shown superiority compared with endoscopic and histological remission for predicting adverse outcomes.9 Furthermore, pCLE has demonstrated the ability to assess and characterise of IBD-associated dysplasia. However, while an initial study by Kiesslich et al showed a 4.75 times higher neoplasia detection rate with pCLE compared with conventional colonoscopy,48 a subsequent study was terminated early due to critical equipment failure.49 Hence, the clinical applicability of this tool is still limited by some practical drawbacks, including the cost of the procedure, lack of codification and reimbursement in some countries, absence of standard of care indications, availability, physician image-interpretation training, medico-legal problems and the role of pathologist.50 Nonetheless, these limitations are relative and future studies will help in solving these barriers, especially when enabled by AI.
Similarly, endocytoscopy, combined with applying topical contrast agents, provides up to 520-fold mucosal magnification, enabling the assessment of barrier features, including crypt architecture, cellular and nuclear morphology, and presence and characterisation of inflammatory infiltrate.51 Endocytoscopy-based scores have been recently developed to evaluate the ileal and colonic barriers in IBD, showing promising potential in predicting outcomes independently and when combined with assessing barrier proteins.52 Main studies evaluating the ability of advanced endoscopic tools to assess the intestinal barrier and predict outcomes in IBD are summarised in table 1.
New advanced endoscopic tools to assess intestinal barrier and predict outcomes in IBD
Finally, preliminary data have shown both techniques’ promising ability to identify in vivo histological features suggestive of IBD-associated dysplasia and CRC, providing insights for diagnosis and treatment planning.53
‘Molecular’ and ‘ultrastructural’ assessment
Innovative imaging technology platforms are being developed for a comprehensive molecular and ultrastructural assessment of intestinal barrier integrity and dysfunction. Though extensively used for immune-oncology-based tissue ultrastructure assessment,54 these cutting-edge techniques are increasingly being explored in gastrointestinal research, especially in the realm of IBD.52 These advanced imaging platforms can accurately detect subtle changes in molecular interactions, immune-based inflammatory pathophysiology and tissue structure, with high levels of sensitivity and specificity. Multispectral imaging of multiplex immunofluorescence,11 upconversion nanoparticles-based biophotonics,12 digital spatial profiling,13 optical spectroscopy14 and imaging mass cytometry15 are increasingly gaining relevance for predicting outcomes, evaluating responses to therapies and discovering novel treatment targets in IBD and CRC. Multispectral imaging platforms are especially being explored due to the deep insights into tissue microenvironment. They allow for the exploration of biomarkers, cell-to-cell spatial interactions and changes in inflammatory tissue damage-mediated expression levels of TJ and AJ proteins, such as ZO-1, claudin-2 and JAM-A.52
These technologies offer a reliable and reproducible workflow suitable for high-throughput clinical and diagnostic use. Furthermore, the spatial biology-based molecular exploration provides insights into the physiological foundations of intestinal disease pathogenesis, leading to the discovery of novel treatment targets. Hence, these technologies offer exciting new opportunities for in-depth analysis of the complex biology of the gut barrier and the gut-brain axis. Table 2 summarises the latest advancements in imaging technology for the spatial analysis of tissue ultrastructure.
Latest advancements in imaging technology platforms being used for spatial analysis of tissue ultrastructure
Artificial intelligence
Integrating AI and computer vision into the medical field has opened new vistas for diagnosing and treating various conditions in endoscopy, histology and intestinal barrier assessment. The automated computerisation of in vivo and ex vivo imaging marks a significant leap towards defining barrier healing.
The computer-based analysis of pCLE features, including vessel tortuosity, crypt morphology and fluorescein leakage, enables objective and quantitative assessment of structural and functional damage to the barrier.46 Furthermore, the sophisticated pCLE-based barrier assessment has the potential to clarify drug transport across the intestinal barrier and aid clinical translation in drug development, in particular biologics in IBD and molecularly targeted therapies in CRC.55 Ex vivo computer-aided pCLE molecular imaging with fluorescein-conjugated biologics (infliximab and vedolizumab), studying pretreatment binding to biological agents, was correlated with an augmented probability of treatment efficacy.46 Similarly, an ex vivo convolution neural network-based approach has been developed for analysing label-free leucocyte trafficking dynamics across vascular barriers.56
Moreover, several studies integrating AI and computer-aided diagnosis with virtual chromoendoscopy and ultra-high magnification endoscopy have demonstrated that AI-based analysis of microvascular architecture on colorectal mucosa can predict histological inflammatory activity, as well as the risk of subsequent relapse in UC patients.57–59 Fusing AI and advanced endoscopic technology may open in vivo vascular barrier assessment.
Despite the application of AI in barrier assessment for IBD-associated neoplasia being in its infancy, a recent development in AI technology applied to endoscopy shows promise. This novel AI model has been validated for detecting and characterising IBD-associated lesions, achieving a lesion detection rate of 90.4%, with good sensitivity and specificity.60 Although this represents only an initial endeavour, it provides optimism for future application in enhancing the characterisation and management of IBD-associated neoplasia.
The integration of AI with histopathology has garnered significant interest because of its ability to detect abnormalities not discernible to the human eye. This fusion facilitates exact forecasts of recurrence in IBD and metastasis and prognosis in CRC.61–63 Goblet cells, essential for mucin production in the intestinal lining, play a pivotal role in IBD. Their dysfunction or depletion contributes to impaired mucosal barrier integrity, increasing susceptibility to IBD flare due to compromised epithelial protection against pathogens and irritants. A deep-learning-based automated quantification of goblet cell mucus using digital whole slide imaging of patients with UC has shown promise in correlating the amount of goblet cell mucus and future relapse risk.64
In addition, AI can be applied to ultrastructural imaging, advancing diagnosis and prognosis assessment. For instance, the unique combination of Raman spectroscopy and advanced machine learning was promising for the non-invasive and rapid classification of IBD.14 Furthermore, the automated quantitative analysis of junctional molecular component protein markers may serve as a novel, objective ground truth for identifying barrier dysfunction. Recently, the automated quantification of TJ protein expression, including claudin-2, occludin and JAM-A, through multiplex immunofluorescence, using the inForm Akoya Biosciences digital multiplex, has enabled significant prediction of adverse outcomes in patients with IBD.52
The application of AI extends beyond the precise diagnosis of intestinal barriers, encompassing the exploration of barrier-protective therapy. Using machine learning model, epithelial barrier-related gene clusters that can predict therapeutic response have been identified, resulting in PRKAB1- the β1 subunit of the metabolic master regulator, AMPK—as a promising gut barrier-protective target.55 This AI-assisted approach has the potential to identify a first-in-class gut barrier-protective agent and predict candidate agents’ phase-III success.
In conclusion, while promising, the preliminary evidence showing the potential of AI-enabled advanced endoscopy and ultrastructural imaging to assess intestinal barrier14 46 52 remains limited and preliminary. Therefore, caution is warranted in interpreting these findings while further refinements are taking place. Nonetheless, integrating AI, which facilitates the combination of advanced endoscopy with molecular imaging and digital pathology, presents the potential to refine our intestinal barrier assessment (see figure 3). AI can contribute to the improved accuracy in diagnosing and predicting IBD, thereby enhancing long-term outcomes and survival rates and personalising patient care, heralding a new era in personalised medicine.
Advanced tools for assessing the intestinal barrier. This figure shows various innovative tools available for assessing the intestinal barrier. Endocytoscopy and probe-based confocal laser endomicroscopy (pCLE) enable evaluation of the barrier at the cellular level (‘cellular‘ barrier), while cutting-edge laboratory techniques, like multispectral imaging of multiplex immunofluorescence depicted here, go deep into the ultrastructural level (‘molecular’ barrier). Additionally, the central circle highlights some artificial intelligence (AI) applications in barrier assessment, including computer-aided imaging analysis of pCLE and automated evaluation of multiplex immunofluorescence for assessing tight junction expressions. AI can assist in integrating these tools, offering a precise, real-time and standardised barrier assessment, thereby facilitating a comprehensive understanding of the gut-brain axis and identifying promising therapeutic targets and agents. Created with ‘Biorender.com’.
New perspectives for IBD and CRC management
The advanced tools available for precise assessment of the intestinal barrier hold promise for evaluating barrier healing as a therapeutic goal and for developing novel therapeutic options in IBD. Despite numerous drugs that have emerged for treating IBD, encompassing new biological and targeted oral therapies, the response to treatment remains unsatisfactory, with an existing therapeutic ceiling hovering around 30% for IBD.65 The failure of existing therapies fuels disease progression, diminishes patient quality of life, increases healthcare costs and contributes to high mortality. This emphasises the pressing need for a more profound understanding of the underlying pathogenic mechanisms, coupled with a holistic and multimodal patient stratification approach. Such efforts are crucial for identifying novel and effective therapeutic targets, paving the way towards personalised therapeutic management approaches.
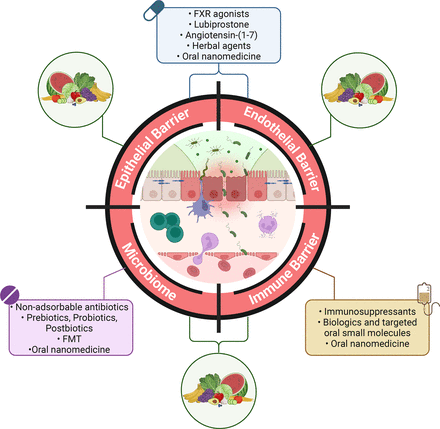
Given the pivotal role of the intestinal barrier and the gut-brain axis in the development and progression of IBD, as well as in IBD-related neuropsychiatric, neurodegenerative, and liver disorders, and colitis-associated CRC, there is growing optimism and hope regarding their potential as novel therapeutic targets (figure 4).
Intestinal barrier as therapeutic target in inflammatory bowel disease. This schematic illustration depicts various compounds currently available or under investigation for targeting different components of the intestinal barrier. Agents capable of targeting the epithelial, endothelial, immune barriers and microbiome are listed. Additionally, the potential of dietary interventions to impact multiple barrier targets is highlighted. Created with ‘Biorender.com’. FMT, faecal microbial transplant; FXR, farnesoid X receptor.
Impact of available medications on intestinal barrier
It remains to be seen whether agents currently employed in clinical practice can effectively target and restore the integrity of the intestinal barrier. Recently, the anti-IL-23 agent guselkumab has demonstrated promising efficacy in promoting epithelial barrier repair, as evidenced by the increase in epithelial cell population found in transcriptomic analysis among patients with moderately to severely active UC.66 Similarly, in the same patient population, ozanimod, a modulator of the sphingosine-1-phosphate receptor 1, has shown potential in modulating gut angiogenesis, promoting intestinal endothelial cells migration, proliferation and pro-angiogenic responses in vitro, suggesting a capacity to address vascular barrier impairment.67 Moreover, the combination of vedolizumab, an antilymphocyte trafficking drug inhibiting α4β7-integrin, with Janus kinase inhibitors has yielded promising results in modulating intestinal barrier disruption in in silico CD models.68 Nonetheless, whether the improvement and restoration of intestinal barrier function by these drugs stem from a specific effect on the barrier itself or represent an indirect and secondary consequence of their actions on inflammatory and immune pathways has not been clarified yet.
Targeting intestinal microbiome
In exploratory human and animal model experiments, targeting intestinal microbiome has shown potential to restore barrier defects,69–71 despite further human trials are needed. We now have at our disposal agents capable of altering the composition of the intestinal flora, such as non-adsorbable antibiotics; we can enhance beneficial populations or metabolites through prebiotics, probiotics and postbiotics, including SCFAs; where necessary, the entire replacement of intestinal microbiota through faecal transplantation is also an option.72 Noteworthy is the reported ability of numerous probiotic strains or their postbiotics, including those from the Lactobacillus and Bifidobacterium species, to promote mucin secretion, upregulate TJ protein expression and promote epithelial restitution.73 The modulation of the intestinal barrier could represent a key mechanism contributing to the promising ability of probiotics/postbiotics to prevent and treat colitis-associated neoplasia. Nonetheless, other mechanisms may play a significant role, including probiotics inhibiting cancer cell proliferation, antagonising oxidative stress and enhancing host immunity, thereby modulating response to immunotherapy.74
In IBD, supplementation with probiotics and SCFAs, such as acetate, propionate and butyrate, has shown beneficial effects, primarily attributed to restoring intestinal barrier structure and function.69–71 Studies conducted in cell lines and primary cell models have demonstrated SCFA’s ability to promote epithelial barrier function by inducing the expression of genes encoding TJs and transcription factors, such as STAT3 and SP1. Additionally, SCFAs regulate the epithelial-microbiome interaction by promoting the production of antimicrobial peptides by intestinal epithelial cells. Notably, butyrate has exhibited anti-inflammatory effects by modulating inflammatory cytokine pathways and immune cells, including the modulation of macrophage polarisation. Similarly, postbiotics have shown the ability to protect the intestinal epithelial and vascular barrier even towards harmful enteropathogens, such as Salmonella typhimurium, through the upregulation of TJ proteins and the control of PV-1 expression by endothelial cells.73 While these findings are promising, further research is needed to fully understand the potential of barrier modulation targeting the microbiome, including the speculation of faecal microbial transplant in IBD treatment, although its effectiveness in inducing long term remission remains controversial.
The intricate and not entirely understood interaction between the host and the microbiome poses the main limitation of these approaches. Nonetheless, the thorough examination of spatial characteristics of host-gut microbiota interactions enabled by novel technologies, such as multi-OMICs, holds promise in untangling this puzzle.75 76 These novel approaches can help in profiling host proteins and microbes, enabling in clinical practice the selection of appropriate agents to modulate the intestinal barrier.
Targeting the epithelial and vascular barrier components
An intriguing approach is the modulation of the intestinal barrier through farnesoid X receptor (FXR) agonists, such as obeticholic acid.77 These molecules have shown a remarkable ability to preserve intestinal epithelial and vascular intestinal barriers in IBD, preventing bacterial translocation in experimental models. Similarly, targeting the FXR/βKlotho/fibroblast growth factors pathway has shown promise to protect the intestinal barrier and prevent CRC by improving TJ markers, inflammation and bile acid levels in mouse models.78 Another compelling molecule is the ClC-2 chloride channel activator, lubiprostone, which has garnered attention for its ability for enhancing barrier properties, particularly in CD, as shown by the increased ion transport, improved permeability and increased TJ expression in vitro.79 Moreover, considering the crucial role of ACE in maintaining intestinal barrier homeostasis, treatment with angiotensin-(1-7) has shown positive results in restoring gut barrier integrity in colitis by modulating the layer of intestinal stem cells and restructuring the gut microbiome.80 Additionally, several herbal agents, including citrus flavonoids such as naringin and hesperidin, as well as the isoquinoline alkaloid berberine, have been studied for their effects on the intestinal barrier, particularly in modulating TJs, showing potential to alleviate inflammation and CRC tumorigenesis.81 However, their effectiveness and applicability in clinical practice remain unclear.
Oral nanomedicine
Advancements in this field include the development of oral NPs designed to selectively deliver drugs and target different components of the intestinal barrier.82 NPs have shown the ability to directly modulate and restore the intestinal epithelium, as demonstrated by studies using KPV peptide-based NPs, anti-intercellular adhesion molecule-1 antibody-coated polystyrene NPs, and anti-transferrin receptor-conjugated NPs. Recently, bilirubin-attached low-molecular-weight, water-soluble chitosan NPs have demonstrated promising therapeutic efficacy in colitis mouse models by promoting the restoration of intestinal barrier, mucosal immunity and gut microbiome.83 Furthermore, oral nanomedicine can selectively modulate the immune system in the lamina propria, with neutrophils and macrophages being potential therapeutic targets. NPs composed of mannosylated bioreducible cationic polymer, sodium triphosphate and TNF-α siRNA have been developed to target macrophage surface receptors, showing strong anti-inflammatory ability in colitis models.84 Similarly, Ly6C+ inflammatory leucocytes have shown promise as targets for nanomedicine, with various lipid-based NPs being developed in recent years to modulate mRNA expression in these cells selectively.85 Finally, oral nanomedicine can precisely manipulate the gut microbiome, exhibiting anti-inflammatory and, notably, anticancer activity. Oral administration of irinotecan-loaded dextran hybrid nanosystem has demonstrated a promising ability to modulate the gut microbiota, targeting the pro-tumorous F. nucleatum and antineoplastic butyrate-producing bacteria, inspiring new approaches for the treatment of CRC.86
Dietary interventions
Dietary components, often associated with lifestyle habits, have a profound impact on our intestinal microbiome and the maintenance of intestinal barrier integrity and functionality.27 Industrial food additives and dietary components, such as refined sugars and saturated fats, have been linked to impaired intestinal barrier function and increasing incidence of immune-related disorders.87 On the other hand, the Mediterranean diet, rich in fermentable fibres, polyphenols, resveratrol, lycopene and omega-3 fatty acids, has consistently demonstrated significant benefits for both IBD and CRC. It enhances gut barrier integrity, modulates TJ protein expression, and promotes a beneficial gut microbiota composition.88 Recently, supplementation with dietary selenium has shown promise in modifying CRC tumorigenesis by modulating intestinal barrier integrity.89 Additionally, emerging research suggests a significant role for dietary sphingolipids (SLs) in regulating intestinal homeostasis and modulating barrier function, with potential therapeutic implications for both IBD and CRC.90 91 Various dietary SLs, notably sphingomyelin, sphingosine, ceramide, sphingosine-1-phosphate and ceramide-1-phosphate, have shown preliminary promise in regulating epithelial cell proliferation and differentiation along the crypt-villus axis. They also can interact with TJs, modulate the composition of the mucus layer, and regulate the formation of plasma membrane lipid rafts and related inflammatory signal transduction. Furthermore, SLs have demonstrated the ability to target the gut-brain axis through the modulation of mast cells, whose degranulation and interaction with central nervous system cells are considered potential links between the gut and the brain. Finally, SLs may play a role in modulating the intestinal microbiome through microbial assimilation of dietary SLs, especially in SL-producing bacteria, such as those of the Bacteroides genus.
Notably, the complex host-microbiome-food interplay can directly impact the effect of dietary interventions on the barrier. Metabolites produced by commensal organisms, including in response to diet, can affect host metabolic processes, potentially leading to protective or pathogenic consequences.92 A multi-OMIC phenotyping can provide deeper insights into the dynamic interaction among diet, the microbiome and the gut and circulating metabolome, elucidating how dietary compounds modulate microbiome composition and alter host metabolism. These approaches are promising for tailoring dietary therapeutic interventions to individual patients.93
In conclusion, interventions aimed at modulating the intestinal barrier and the gut-brain axis, targeting the microbiome and the epithelial and vascular barrier components, represent promising avenues for personalised therapeutic interventions, leading to improved outcomes in patients with IBD and colitis-associated neoplasia. Nonetheless, available data are mainly from animal studies and in vitro research, and to date, no drug has been approved by the US Food and Drug Administration or the European Medicines Agency for modulating the barrier. Dietary interventions offer a complementary approach to bolstering intestinal barrier function and enhancing disease management strategies.
Future directions
Our deepened understanding of IBD pathogenesis has shed light on the pivotal role of the intestinal barrier and its intricate interplay with dietary factors and the microbiome in shaping the trajectory of IBD. Barrier impairment and microbial translocation are central to IBD pathogenesis, with implications extending systemically through the gut-brain axis, leading to neurological and liver disorders and CRC metastasis. Yet, our knowledge of these components and their interactions still needs to be revised.
Advancements in real-time endoscopic tools and molecular technology offer precise and deep intestinal barrier assessment. Furthermore, functional MRI shows promise for in vivo real-time assessment of the brain barrier. Integrating these imaging advances with AI holds the potential to finally connect the dots and fill in the missing pieces of the gut-brain axis, aiding in the assessment of barrier healing and uncovering new therapeutic targets and agents.
Currently, available drugs primarily target the immune component of the barrier. Novel agents and lifestyle interventions, with the pivotal role of diet, capable of modulating the intestinal barrier, the immune system, the microbiome, and consequently the gut-brain axis, hold immense potential to revolutionise the therapeutic landscape and improve outcomes for patients with IBD.
By embracing a comprehensive approach to the gut-brain axis, we can aspire to reshape the trajectory of IBD (box 1). This offers hope for a future where effective treatment strategies prevent barrier damage and restore barrier integrity, thus promoting optimal gastrointestinal and ‘holistic’ systemic homeostasis.
Intestinal barrier: opening the door of precision medicine in inflammatory bowel disease (IBD) and colitis-associated neoplasia
Clinical applications
Intestinal barrier healing: potential therapeutic avenue in clinical trials and clinical practice for deep patient stratification for mucosal healing and precise prediction of long-term disease outcomes.
Advanced endoscopic techniques: available technologies to assess barrier healing in real time and forecast disease course, potentially guiding therapeutic decisions.
Advanced molecular imaging: provides a comprehensive and quantitative characterisation of the intestinal barrier, aiding in patient stratification and personalised treatment strategies.
Artificial intelligence: applied to endoscopy and molecular imaging holds potential for standardising objectively barrier assessment and outcome prediction. It can also aid in the identification of gut barrier-protective therapeutic targets and predict the success of barrier-targeting candidates in trials.
Targeting intestinal barrier: promising agents (eg, guselkumab, tofacitinib, ozanimod, obeticholic acid and lubiprostone) have shown efficacy in mouse models and in vitro studies aimed at restoring the barrier, potentially offering a significant advancement in IBD management.
Emerging research directions
The chicken or the egg dilemma: preliminary evidence suggest that barrier dysfunction is an early event in IBD pathogenesis; further prospective long-term studies are needed to address this question and potentially consider targeting barrier as a preventive or early therapeutic strategy in IBD.
Novel insights into intestinal barrier: the full composition and functionality of the intestinal barrier remain incompletely understood. Novel imaging and molecular techniques hold promise in providing deeper insights into the function of barrier, aiding the identification of promising therapeutic targets.
Novel biomarker discovery: identification of non-invasive epithelial and vascular barrier-related biomarkers, valuable to track disease progression, forecast outcomes, anticipate complications and guide therapeutic decisions.
Gut-brain axis: the connection between brain and gut, mediated by microbiome ‘leaking’, garnered increasing attention. The AI-aided combination of advanced endoscopy, molecular imaging and functional cross-sectional brain barrier imaging represents a step forward in untangling the intricate puzzle of the gut-brain interaction.
Host-microbiome-food interaction: novel multi-OMIC approaches can assist in profiling gut microbiome and bacterial metabolites, identifying mechanisms of food-microbiome interplay and unravelling host metabolic process. Shedding light on these interactions will help to identify novel therapeutic targets.
Supplemental material
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
GS and SM contributed equally.
Contributors Conceptualisation: MI, SG, GS, SM; writing—original draft preparation: GS, SM, JM, IZ, YM; writing—review and editing: MI, SG, GS, SM; supervision: MI, SG, MRA, JFC, ADS, MR. All authors have read and agreed to the published version of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.